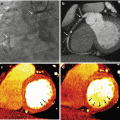
Fig. 6.1
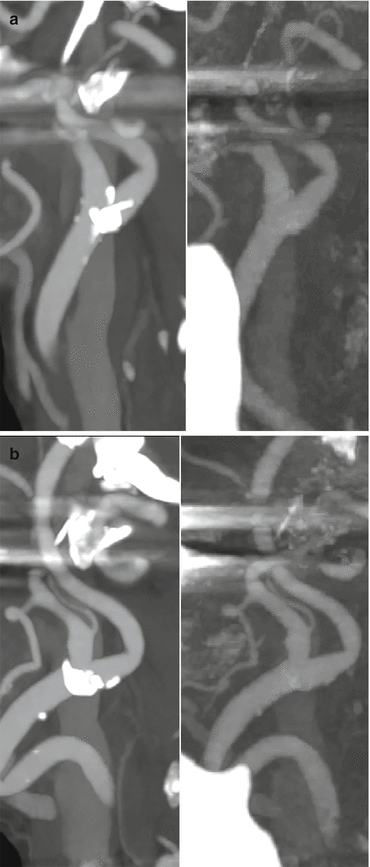
Dual energy CT with calculated MCE sagittal images of the cervical spine in a patient with prior C2-C5 anterior cervical fusion, corpectomy and cage placement. Hardware associated streak artifact is decreased at 92 keV (b) when compared to 70 keV (a)
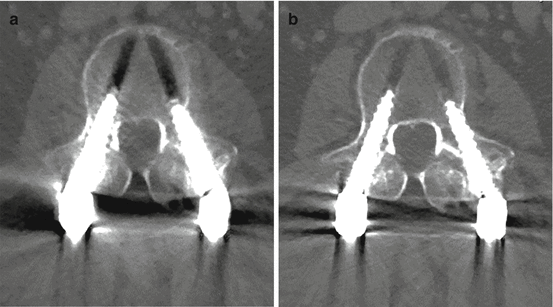
Fig. 6.2
(a, b) MCE imaging in spine instrumentation. 70 keV image left and 110 keV image right. Note the striking reduction in BHS on the balanced image at higher energy
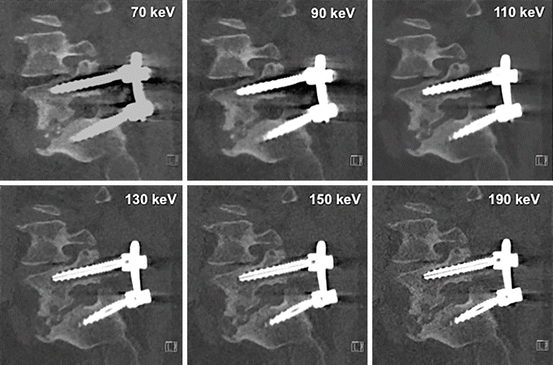
Fig. 6.3
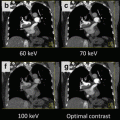
Monoenergetic imaging – windowing by energy. Note that as energy level increases, hardware visualization becomes more discrete with less blooming and better appreciation of the internal screw architecture. Low contrast detectability drops at the highest energy levels and noise increases thus 110 keV is best for routine interrogation. The highest energy levels may add incremental information about the hardware itself and the immediately surrounding anchoring bone
Material Basis Imaging
Material decomposition can assist in bone removal, calcium/hydroxyapatite subtraction and quantification of iodine or hemorrhage within a given tissue. In routine neurological imaging this is used to differentiate blood, calcium and iodinated contrast. These materials, differing by atomic number, are presented in complementary pairs, e.g., water-iodine, iodine-calcium and water-calcium. By convention the output highlights one material within the tissue while suppressing the other (Figs. 6.4 and 6.5). Overlays of atomic number signature can also be placed atop standard medical images to highlight the presence of elements such as iodine in enhancing lesions (Fig. 6.6).
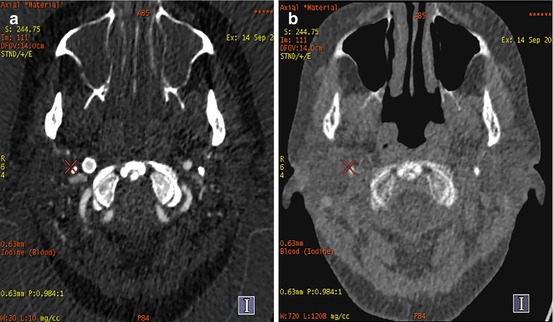
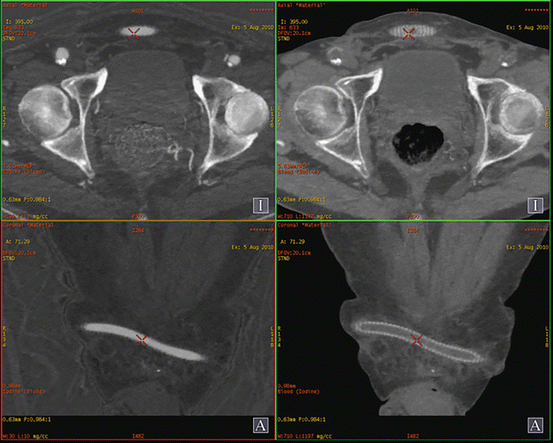
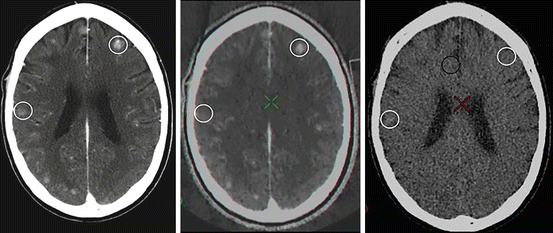

Fig. 6.4
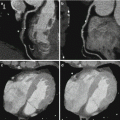
Materials basis imaging. Axial DE CTA of the neck with material basis post-processing in a patient with previous placement of a right internal carotid artery stent for symptomatic stenosis. Figure (a) highlights the contribution of iodine to the image whereas figure (b) suppresses the contribution of iodine allowing depiction of the placed right internal carotid artery stent

Fig. 6.5
Materials basis imaging. Axial and reformatted coronal images in a patient with a femoral-femoral cross over bypass graft for right iliac artery occlusion. The “blended” dataset is seen on the left with the images on the right representing the “virtual non-contrast” or iodine suppressed images obtained from the same acquisition

Fig. 6.6
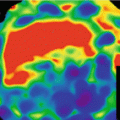
Material basis imaging from left: MCE image at 65 keV, iodine basis image and iodine suppressed image. Metastatic disease with enhancing and hemorrhagic lesions. Lesion at 2 o’clock seen on MCE and iodine images consistent with enhancing metastasis. Lesion at 9 o’clock seen on MCE and iodine suppressed images consistent with hemorrhage
Head and Neck CTA
CTA has largely replaced catheter angiography in the initial evaluation of patients with suspected subarachnoid, subdural and parenchymal hemorrhage as well as ischemic stroke. It is more readily available, more comfortable for patients than MRI/MRA and devoid of the user dependent and anatomic limitations of vascular ultrasound. However, despite the advantages CTA provides, challenges exist. Conventional polychromatic CT is prone to scatter and streak/beam-hardening artifact at and around the skull base and about metal implants such as aneurysm clips and coils. CTA is also limited in assessing lumen dimensions in the presence of heavily calcified plaques, particularly when associated with significant stenosis. DESCT improves assessment of head and neck vessels by minimization of streak and beam hardening artifacts and boosted depiction of enhancement over conventional CT techniques (Fig. 6.7). DESCT is sensitive to the varying behavior and appearance of materials of differing atomic number, such as water, hemorrhage, iodine and calcium, across a range of X-ray energies. This improves the differentiation of materials and allows novel subtraction or suppression based imaging techniques. CTA studies of the head and neck performed with DESCT bone removal have been shown to improve image quality and decrease reading time [2–5].
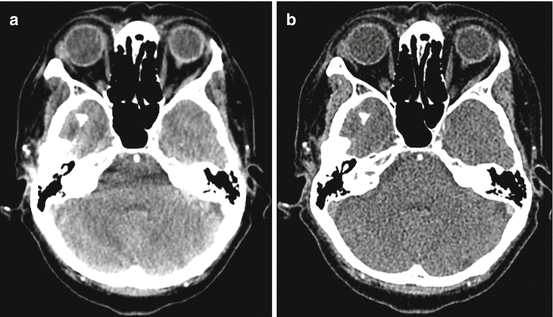

Fig. 6.7
Axial dual energy CTA of the head through the posterior fossa at blended 80 kvP (a) and when processed as monochromatic monoenergetic 80 keV (b) images. There is a significant decrease in skull base related beam hardening artifact in the MCE processed images with a trade off in slightly increased image noise
Clinical Applications
Intracranial Hemorrhage, Contrast Enhancement and Calcification (Virtual Unenhanced Brain CT)
In patients with suspected subarachnoid hemorrhage or known parenchymal hemorrhage the routine non contrast study may be omitted in favor of DESCT based virtual unenhanced scan. There are two fundamental methods of obtaining a virtual unenhanced image with DESCT.
The contribution of iodine to an individual voxel can be estimated and subtracted from the final product resulting in a “virtual” unenhanced CT. At 80 kVp, the x-ray beam is more significantly attenuated by iodine compared to 140 kVp due to its high atomic weight and the more prominent contribution of the photoelectric effect to beam attenuation. The remaining intracranial soft tissues have a far less marked difference in their attenuation between 80 and 140 kVp due to their low atomic number. Following this principle, material basis images can be created to subtract or depict the contribution of iodine to any given voxel providing an image that shows only enhancement or alternatively bone, brain parenchyma, CSF and blood products but no enhancement (Fig. 6.6).
Iodine conspicuity is increased at lower levels of MCE, which more closely approximate the k-edge of iodine (33.2 keV). Lower energy levels improve visualization of parenchymal/lesion enhancement and intraluminal contrast at the expense of some increase in image noise (Fig. 6.8). Windowing by energy can confirm the presence of enhancement in subtle cases and lower energy choices can be made if contrast load is reduced in situations where there is concern about contrast induced nephropathy. The highest energy levels effectively suppress the appearance of iodine providing a second method of deriving virtual unenhanced imaging from a contrast enhanced scan. MCE has been shown to be effective in differentiation of enhancement from acute or sub-acute brain hemorrhage [6].
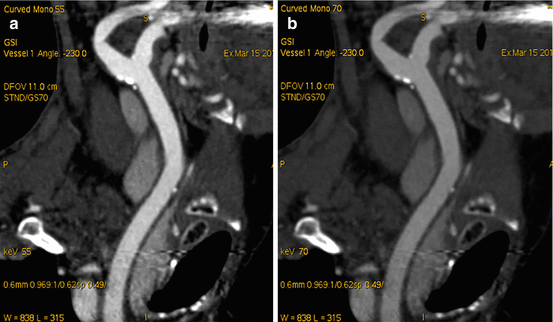

Fig. 6.8
Coronal oblique MIP reformatted CTA of the neck in a patient under investigation for carotid disease. Improved conspicuity of iodine at the lower selected monoenergetic monochromatic energy level of 55 keV (a) compared to 70 keV (b)
Classification/Characterization of Carotid Plaque
Enriched tissue differentiation with bone and calcium subtraction improves vessel segmentation and stenosis evaluation. Single energy CTA is challenged in the presence of high-grade stenosis when plaque is heavily calcified. Despite the use of limited volume and curved planar reformations the blooming of CT dense plaque hampers delineation of the interface between plaque and lumen. This interferes with definitive assessment of lumen stenosis particularly at the carotid bifurcations and siphons. The bone of the skull base, and thoracic outlet also obscure vascular anatomy. This is only partially ameliorated by segmentation techniques based on Hounsfield unit (HU) based thresholding.
DESCT can be used to overcome some of the limitations of single source CTA imaging. Tissue assessment based on behavior across the spectrum of energies allows segmentation based on atomic number. Interactive ‘windowing’ based on energy (keV) can be a powerful complement to traditional HU based techniques by more effectively manipulating the appearance of iodine based contrast with respect to adjacent tissues. Iodine based and calcium/hydroxyapatite suppressed renderings can provide DSA “like” subtraction MIP images [3] (Figs. 6.9 and 6.10). Despite promise, limitations of this technique have been described. There can be overestimation of stenosis and pseudo-occlusions in high-grade calcific disease. Persistent blooming and beam hardening effects of the calcified plaque may hinder the differentiation calcium from adjacent luminal contrast in small vessel channels. The resultant obscuration of the vessel lumen may contribute to an overestimation of stenosis [7]. Appropriate manipulation of isotropic source data (monoenergetic or blended) on a 3D workstation will overcome many of these limitations. The unique contributions of DESCT to CTA provide a potentially powerful complement to routine evaluations of neurovascular disease [8].