11 Duplex assessment of aneurysms and endovascular repair
INTRODUCTION
True aneurysms are abnormal dilations of arteries. The term ectasia is often used to describe a moderate dilation of arteries. The abdominal aorta is one of the commonest sites for aneurysms to occur. The main risk of abdominal aortic aneurysms (AAA) is rupture, which is fatal in most cases. In the USA rupture of an AAA is the 14th commonest cause of death and is estimated to kill approximately 10 000 people per year (Birkmeyer & Upchurch 2007). In England and Wales, this figure is approximately 5000 people (Office for National Statistics 2006). Men over the age of 65 years are the most common group to be affected. Ultrasound can detect almost all AAAs and, when screening is combined with elective surgery, the mortality associated with the disease is almost halved (Ashton et al. 2002). A recent analysis of four large, randomized clinical trials has also confirmed that population-based screening substantially reduces AAA-related mortality in selected patient groups (Fleming et al. 2005) In the USA, the US Preventive Services Task Force (2005) and a consortium of leading professional organizations recommend one-time screening with abdominal ultrasonography for all men aged 65–75 years who have ever smoked. In the UK, a national screening program should be under way by the time this edition is published.
Ultrasound is the obvious modality for screening as it is a rapid, cheap, and simple noninvasive method of detecting aneurysms and can be used for serial investigations to monitor any increase in size of small aneurysms. However, if surgical intervention is being considered, other imaging techniques, such as computed tomography (CT) and magnetic resonance imaging (MRI), are required to demonstrate the relationship of an aneurysm to major branches and other structures within the body. In the past, treatment of AAA was by open surgery but nowadays approximately 50% of patients are treated by the less invasive technique of endovascular aortic aneurysm repair (EVAR), where a stent graft is inserted via the femoral arteries. Endovascular repair can also be used to treat aneurysms in other areas of the body. This chapter concentrates on ultrasound scanning of aortic aneurysms and surveillance of EVAR procedures but also considers the assessment of aneurysms in other areas of the peripheral circulation.
Definition of an aneurysm
It has been suggested that an aneurysm is a permanent localized dilation of an artery having at least a 50% increase in diameter compared to the normal expected diameter (Johnston et al. 1991). Ectasia is characterized by a diameter increase <50% of the normal expected diameter. It is worth remembering that there is considerable variability in the normal diameter of arteries among individuals, and this will be dependent on factors such as physical size, sex, and age.
ANATOMY OF THE ABDOMINAL AORTA
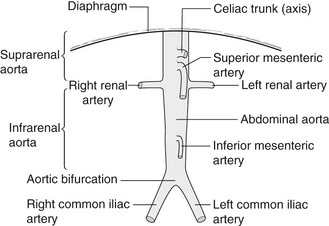
The abdominal aorta commences at the level of the diaphragm and lies just in front of the spine. It descends slightly to the left of the midline to the level of the fourth lumbar vertebra, where it divides into the left and right common iliac arteries (Fig. 11.1). It tapers slightly as it descends, owing to the large branches it gives off. Major branches of the aorta that can be easily identified with ultrasound include the celiac axis and superior mesenteric artery (SMA) (Fig. 11.2). These can act as important reference points when determining the upper limit of an aneurysm. Visualization of the inferior mesenteric artery is variable. The vena cava lies to the right of the aorta and may assume a variety of shapes, especially in the presence of an aneurysm, and commonly appears ‘flattened’ when compared to the circular shape of the aorta.
PATHOLOGY OF ANEURYSMS
The mechanism of aneurysm development is uncertain but may involve a multifactorial process leading to the destruction of aortic wall connective tissue. There is evidence that increased local production of enzymes capable of degrading elastic fibers as well as interstitial collagens is associated with AAA (Wassef et al. 2001). The lumen of an aneurysm is often lined with large amounts of thrombus that can be a potential source of emboli. This is also why arteriograms, which only demonstrate the flow lumen, are not accurate for estimating the true diameter of an aneurysm, as the flow lumen can be significantly smaller than the diameter of the entire vessel. Aortic aneurysms can also extend into the iliac arteries. Some aortic aneurysms are involved in an inflammatory process, with marked periaortic fibrosis surrounding the aorta making surgical resection difficult (see Figure 11.12D). Aneurysms can also be caused by a variety of infections, such as bacterial endocarditis, and are termed mycotic aneurysms. These can occur anywhere in the body.
ANEURYSM SHAPES AND TYPES
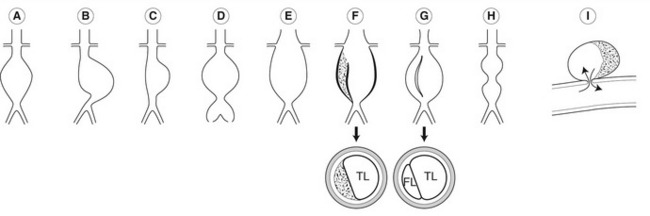
Aneurysms vary considerably in shape and size (Fig. 11.3). Most aneurysms are fusiform in shape and there is uniform dilation across the entire cross-section of the vessel. Saccular aneurysms exhibit a typical localized bulging of the wall. Dissecting aneurysms occur due to a disruption of the intimal lining of the vessel, allowing blood to enter the subintimal space. This can result in the stripping of the intima, and sometimes of the media, from the artery wall. If the aorta partially dissects, large amounts of thrombus may be seen in the subintimal space (Fig. 11.3F). If there is a full dissection, a false flow lumen is created and the dissected layer of intima may be seen flapping freely in time with arterial pulsation (Fig. 11.3G). Some aortic dissections are not associated with aneurysms and can start in the chest, extending through the aorta into the iliac arteries. It is possible for aortic branches, such as renal arteries, to be supplied via either lumen. Occasionally, two aneurysmal dilations may be seen along the length of the abdominal aorta, separated by a normal segment of the aorta, which gives rise to a classic “dumb-bell” shape when viewed in longitudinal section (Fig. 11.3H). As the aorta dilates, it also tends to increase in length, producing tortuosity that often shifts the aorta to the left of the midline or deflects it in an anterior direction.
AORTIC ANEURYSMS: SYMPTOMS AND TREATMENT
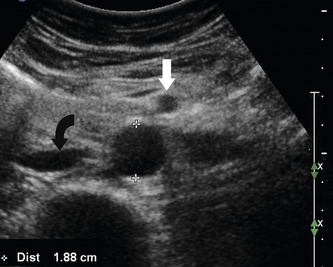
The normal size of the abdominal aorta varies between 1.4 and 2.5 cm in diameter (Johnston et al. 1991) (Fig. 11.2). An aortic diameter slightly above 2.5 cm is considered mildly abnormal or ectatic. A small aortic aneurysm is generally regarded as an aorta having a diameter of 3 cm, and many surgeons will not request serial screening scans unless the aorta reaches this level. However, some vascular units monitor patients with slightly enlarged aortas, especially if the patient is young (<55 years old). As the aorta increases in size, there is a potential for rupture due to increased tension in the arterial wall. The UK Small Aneurysm Trial Participants (1998) demonstrated that the average annual growth rate of aneurysms measuring between 4 and 5.5 cm was 0.33 cm a year. However, rates will vary among individuals and are also dependent on the size of the aneurysm. The prevalence of aortic aneurysms is five to six times greater in men than women (Vardulaki et al. 2000). In addition, there seems to be a strong familial link, with siblings of aneurysm patients having a higher risk of developing an aneurysm compared with the general population.
Clinically, there are usually no symptoms associated with the development of an aortic aneurysm and many are discovered incidentally, during routine examinations or on plain abdominal radiographs. Occasionally, patients present with symptoms of renal hydronephrosis. This is caused by compression of a ureter leading from one of the kidneys by the aneurysm sac and most frequently occurs on the left side. The symptoms associated with aneurysm leakage or rupture include back or abdominal pain and acute shock. Ultrasound is occasionally used to confirm the diagnosis in the emergency room, although the symptoms are usually so acute that emergency surgery is required. However an emergency room scan that excludes an aneurysm can be useful and many emergency physicians have been trained to undertake rapid AAA scanning. The mortality rate for acute rupture of an aortic aneurysm is very high, 65–85% (Kniemeyer et al. 2000), and many patients do not reach hospital alive.
The risk of aortic aneurysm rupture increases with size. The UK Small Aneurysm Trial Participants (1998) found that the mean risk of rupture of aneurysms measuring 4–5.5 cm was 1% per year. However, larger aneurysms carry a higher rate of rupture. A study by Lederle et al. (2002) demonstrated that the average risk of rupture in male patients with a 6−6.9 cm aneurysm was 10% per year and 32% per year for aneurysms measuring more than 7 cm in diameter.
Clearly, there are benefits in detecting aneurysms at an early stage so that serial follow-up can be carried out and elective repair performed if the aneurysm becomes too large. The UK Small Aneurysm Trial Participants (1998) have shown no survival benefit for open repair of aneurysms measuring less than 5.5 cm in diameter compared to ultrasound surveillance and this was also confirmed after a 12-year follow-up analysis of the trial (The UK Small Aneurysm Trial Participants 2007). In the original study, age, sex, or initial aneurysm size did not modify the overall hazard ratio. Therefore, many surgeons will only carry out elective repair if the aneurysm has a diameter of equal to or greater than 5.5 cm, or if there are indications that smaller aneurysms are becoming symptomatic and are at risk of rupturing.
Although aortic aneurysms are much more prevalent in men, there is some evidence that women with aneurysms in the 5–5.9 cm range may be up to four times more likely to undergo rupture compared to men with similar-sized aneurysms (Brown et al. 2003). Further research may prompt a lower threshold for repairing aneurysms in female patients. However, at the present time the mass screening of women does not appear to be cost-effective.
Surgical techniques for aortic aneurysm repair
Open repair
Open repair of aortic aneurysms has been performed for over 30 years and involves a large incision in the abdomen and mobilization of the intestines to expose the aorta. Fortunately, the majority of abdominal aneurysms (approximately 90%) start below the level of the renal arteries (infrarenal aneurysms). This means that surgical clamps, to control the aneurysm, can be positioned below the renal arteries, ensuring that the kidneys are perfused during the operation. Aortic aneurysms that extend above the renal arteries (suprarenal aneurysms) carry a higher rate of perioperative and postoperative complication, as the aorta has to be clamped above the level of the renal arteries and reimplantation of the renal arteries is necessary. Patients can suffer from renal failure following this procedure. This is why it is important that the surgeon be aware of the level of the proximal neck before surgery is performed. Aortic aneurysms are repaired using straight tube grafts unless the aneurysm extends into the iliac arteries, where a bifurcating graft is used. The graft is sutured into position and the sac closed around the graft. Postoperatively, patients normally spend a day or two in intensive care and usually leave hospital 10–14 days after surgery. The elective mortality rate for open repair is in the region of 5%. However, surgically unfit patients have a risk of much higher morbidity and mortality rates.
Endovascular repair
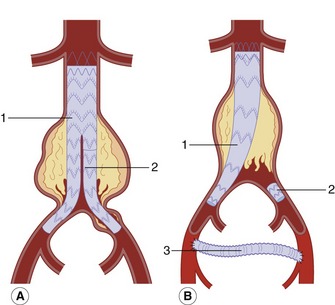
Nowadays, almost all endovascular grafts are bifurcating devices (Fig. 11.4). These are modular systems with the graft supplied in two parts. The bulk of the graft consists of the main body, one complete limb, and the short stump of the second limb. The remaining modular limb is delivered separately via an arteriotomy in the contralateral common femoral artery. The grafts are prepacked on to the delivery catheter during the manufacturing process and retained in place by an outer sheath until deployed in the aorta. During the procedure the femoral artery is surgically exposed, and the catheter containing the main graft is inserted over a guide wire and positioned with the aid of fluoroscopy so that the top of the graft lies just below the renal arteries in the proximal neck. Many of these devices have uncovered metal stents that extend across the renal arteries (suprarenal fixation) to hold the device in place.
The graft is deployed by slowly withdrawing the outer covering sheath. If needed, a soft balloon is inflated to ensure the graft is fully expanded in the proximal neck, just above the sac. Some grafts have hooks at the top that anchor into the aortic wall for further security. The modular limb is then delivered on a separate catheter via the contralateral femoral artery. Under radiographic control it is positioned so that it fits into the stunted limb of the main body and then is fully expanded, using a balloon if necessary, to make a seal. The distal end is then anchored in the common iliac artery. In some cases an aortic uni-iliac device can be inserted if one of the iliac arteries is diseased, occluded, or excessively aneurysmal (Fig. 11.4B).
As the devices are modular, it is possible to add extensions to the limbs to exclude long iliac artery aneurysms. Postoperative recovery is usually very quick, with some patients going home within 2–4 days. However, not all aneurysms are suitable for endovascular repair. This can be due to aneurysm tortuosity, excessive proximal neck diameter, limited proximal neck length, severe iliac artery disease, and marked iliac artery tortuosity. The Endovascular Aneurysm Repair Trial 1 (EVAR 2005) reported a two-thirds reduction in 30-day postoperative mortality compared to open repair. Although endovascular repair appears much less traumatic for the patient, the EVAR 1 trial also found that, by 4 years, 40% of patients who had undergone endovascular repair had suffered a complication and that 20% had required reintervention, including the correction of endoleak. For this reason it has been recommended that patients should undergo lifelong surveillance to detect endoleaks or other graft-related complications (National Institute for Health and Clinical Excellence 2006). An endoleak occurs when blood leaks into the aneurysm sac from the graft or from another source, such as a lumbar or inferior mesenteric artery. In this situation the aneurysm sac can continue to expand and rupture (van Marrewijk et al. 2002; EVAR 2005). The rupture rate in the EVAR 1 trial was 1%. However there is evidence to suggest that some types of endoleak are benign and can be safely left alone unless there is progressive sac expansion. The different types of endoleak and their ultrasound appearances and management are discussed later in this chapter.
PRACTICAL CONSIDERATIONS FOR DUPLEX SCANNING OF AORTIC ANEURYSMS
What information does the physician require?
• The maximum diameter of the aorta
• Any relevant aneurysm features such as shape or position
• Does the aneurysm extend across the iliac bifurcation into the common iliac arteries?
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree