14 Duplex assessment of deep venous thrombosis and upper-limb venous disorders
Epidemiology and pathology of DVT
Practical considerations for duplex assessment of DVT
Deep-vein examination for acute DVT
Scan appearances for the assessment of DVT
Accuracy of duplex scanning for the detection of DVT
Other pathologic conditions that can mimic DVT
Technique for assessing the brachial, axillary and subclavian veins
INTRODUCTION
Deep venous thrombosis (DVT) is a common disorder that can lead to fatal pulmonary embolism (PE); the combined condition is described as venous thromboembolism (VTE). Duplex scanning is considered to be the method of choice for the imaging of DVT, with other imaging techniques reserved for technically incomplete or difficult duplex examinations. Duplex scanning can be used for serial investigations to monitor the progression and outcome of thrombosis. In addition, duplex scanning can be useful for assessing the long-term damage to veins and valve function as a result of chronic postthrombotic syndrome (Haenen et al. 2002). This can lead to the development of lower-limb venous hypertension and possible leg ulceration. This chapter provides a description of duplex scanning techniques for the diagnosis of DVT and also considers other pathologic conditions that may mimic the symptoms of venous thrombosis.
EPIDEMIOLOGY AND PATHOLOGY OF DVT
DVT usually affects the lower-limb veins, but it can also occur in the upper limbs, especially in conjunction with catheter access or malignancy. The published data on the epidemiology of DVT and PE demonstrate some variability, and reported rates of DVT and thromboembolism appear to be partly dependent upon methods of data collection (autopsy records, discharge diagnoses, and so forth) and the patient population studied. A systematic review by Fowkes et al. (2003) indicated an incidence of DVT in the whole general population of approximately 5 per 10 000 per annum. However, the incidence was highly dependent upon age and increased from 2–3 per 10 000 person years at age 30–49 years to 20 per 10 000 person years at age 70–79 years. Around 40% cases of DVT were found to be idiopathic (of unknown cause). The annual rate of PE is somewhere in the region of 6 cases per 10 000 in the general population (Nicolaides et al. 1994). A review by White (2003) indicated that death occurs in approximately 6% of DVT cases and 12% of PE cases within 1 month of diagnosis. Open or healed venous ulceration occurs in around 1% of the general adult population, with a proportion attributed to postthrombotic syndrome (Fowkes et al. 2001). The early detection and treatment of DVT can therefore reduce the subsequent risk of mortality or long-term morbidity.
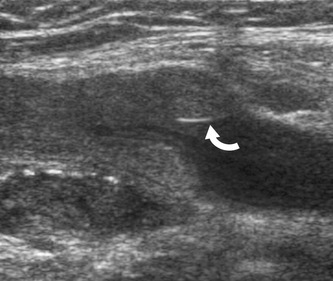
Virchow (1846) described the association between thrombosis in the legs and emboli in the lung. The factors predisposing to thrombosis are described by his famous triad of coagulability of the blood, damage to the vein wall or endothelium, and venous stasis. Venous thrombi are believed to originate in valve cusp pockets (Fig. 14.1) or in the deep venous muscular sinuses, such as the soleal veins. DVT most commonly occurs in the calf veins and can propagate to the proximal veins. In the lower limb the popliteal vein is usually described as a proximal vein. It is not necessary for all the calf veins to be affected in order for proximal propagation to occur. It is believed that approximately 10–20% of calf vein thrombi propagate to the deep veins across and above the knee (Khaw 2002; Labropoulos et al. 2002). This is thought to be associated with an increased risk of PE. Isolated thrombosis of the proximal veins, such as the femoral or iliac veins, is less common and can be due to trauma, surgery, pregnancy, or malignancy. Proximal and distal propagation of thrombus can occur in this situation. Venous stasis can also lead to DVT, and this is why patients undergoing periods of bed rest or immobility are at greater risk of developing thrombosis. In fact hospitalized patients with medical illnesses have a similar risk of VTE as those undergoing major general surgery (Anderson & Spencer 2003).
There is evidence to suggest that long-haul air travel is associated with an increased risk; this risk may be low but, as with VTE generally, additional factors increase the risk. The main risk factors associated with the development of venous thrombosis are shown in Box 14.1. In the early stages of a DVT, it is possible for a large proportion of the clot to be nonadherent to the vein wall. This is termed a free-floating thrombus. In this situation there is a risk of detachment, leading to PE. As thrombus becomes older (7–10 days), it becomes more organized and adherent to the vein wall.
Signs, symptoms, and treatment of DVT
The symptoms of PE include the following:
The clinical diagnosis of DVT is unreliable and inaccurate in up to 50% of cases (Cranley et al. 1976; Beyer & Schellong 2005). However, typical symptoms include the development of acute calf pain associated with localized tenderness, heat, and swelling. The superficial veins may also be dilated. If the thrombosis involves the proximal veins, there may be significant swelling of the calf. Unfortunately, other conditions, such as cellulitis and edema, can mimic the symptoms of DVT. In some cases of DVT the patient may be asymptomatic, especially if the thrombus is small or distal. In extreme cases of DVT the outflow of the limb is so severely reduced that the arterial inflow may become obstructed, leading to venous gangrene. This condition is called phlegmasia cerulea dolens. The foot may appear blackened and the limb swollen and blue, even when elevated.
The high risk of VTE amongst both medical and surgical hospital inpatients has resulted in guidelines from various national and professional bodies as to the best preventive measures (Cayley 2007; National Institute for Clinical Excellence 2007). The prophylaxis of DVT includes the use of mechanical prevention in the form of graduated compression stockings and pneumatic compression devices which increase venous return and therefore reduce the risk of venous stasis. Patients at higher risk may be given low-molecular-weight heparin, which carries a lower risk of bleeding than unfractionated heparin.
The investigation and treatment of isolated calf vein thrombosis remain a contentious issue (Lohr et al. 1991; Meissner et al. 1997; Kearon 2003). It is beyond the scope of this book to consider the debate in any detail, but sonographers should be aware of the controversies surrounding this area; most DVTs start in the calf and most resolve but proximal progression does occur. Other issues in the calf are the extra time to extend the scan from distal popliteal to calf veins, the ability to visualize calf veins adequately, and accuracy of detecting DVT in the calf.
Investigations for diagnosing DVT
Traditionally, X-ray venography was the main test used for the diagnosis of DVT. It involves an injection of a contrast agent into the venous system via a dorsal foot vein. In some cases it proves impossible to cannulate a foot vein, and in some situations patent deep calf veins do not fill with contrast agent (Bjorgell et al. 2000). Currently, duplex scanning is the preferred method of imaging DVT. It is often combined with pre-imaging tests in a defined management pathway or protocol. These pathways have been developed because of financial pressures and a desire to provide a coherent and efficient service. The protocols may be complex and are usually expressed in a diagnostic/treatment algorithm (Fig. 14.2). Algorithms differ in detail (Sampson et al. 2005), with DVT ruled out on low clinical risk and negative D-dimer but sometimes still requiring ultrasound.
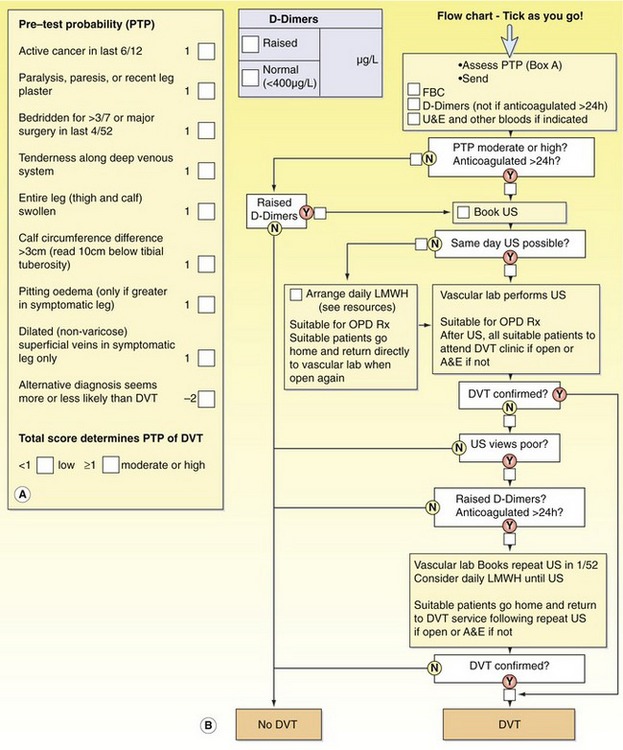
Figure 14.2 An example of a screening protocol for deep venous thrombosis (DVT). (A) Pretest probability score (Wells score). (B) A flow chart for investigations (courtesy of Kings College Hospital, London). FBC, full blood count; U&E, urine and electrolytes; US, ultrasound; LMWH, low-molecular-weight heparin; OPD, outpatients department; A&E, accident and emergency.
Most, but not all, positive diagnosis is by ultrasound. The process normally begins with a clinical assessment, including a risk probability score derived from a set of standard questions. The lower the score, the lower the probability of DVT. An example is given in Figure 14.2, which is based on the Wells score (Wells 2007). The next stage usually involves a biochemical assay to measure D-dimer levels in the blood. D-dimers are products that are formed by the interaction of fibrin, contained in thrombus, and plasmin. Increased levels of D-dimer are associated with the presence of DVT. Unfortunately, increased levels of D-dimer are also found in other conditions, such as malignancy, infection, and trauma. Therefore, the D-dimer test has a high sensitivity but low specificity for the presence of DVT. Despite low specificity, negative predictive values as high as 98% have been reported (Bradley et al. 2000). A negative predictive value indicates the probability that patients will not have the disease in those who have a negative test outcome. (It has been suggested that a combination of a low risk probability score and negative D-dimer test may be useful pre-selection tools to avoid unnecessary duplex examinations; Aschwanden et al. 1999; Orbell et al. 2008.).
Most ultrasound examinations use compression of the vein to confirm patency. This normally involves full examination of the deep veins from the groin to the calf. However, there is evidence that a limited compression test involving two- or three-point compression at the common femoral vein, popliteal vein, and distal popliteal vein (third point) is a method of excluding DVT, in that the cohorts of patients with negative scans and no treatment have low rates of VTE complication at follow-up (Cogo et al. 1998; Khaw 2002). Where the distal veins were not scanned and when clinical suspicion remains or where there are incomplete views of the calf veins, a repeat ultrasound at 1 week may be used to detect progression to distal popliteal vein level.
PRACTICAL CONSIDERATIONS FOR DUPLEX ASSESSMENT OF DVT
The examination room should be at a comfortable ambient temperature to prevent vasoconstriction (>20°C). Wherever possible, the legs should be examined in a dependent position in order to fill and distend the veins. Ideally, the patient should be examined with the legs tilted downward from the head by at least 30° (reverse Trendelenburg position). Alternatively, the patient can be examined in a standing position, with the leg to be examined minimally weight-bearing and the patient holding a hand rail or equivalent for support. The calf veins and popliteal fossa are easier to scan with the legs extended, hanging over the side of the examination table, and the feet resting on a stool. Wherever possible, immobile or sick patients should be tilted into a reverse Trendelenburg position, although there may be situations in which the patient cannot be moved, such as in the intensive care unit.
DEEP-VEIN EXAMINATION FOR ACUTE DVT
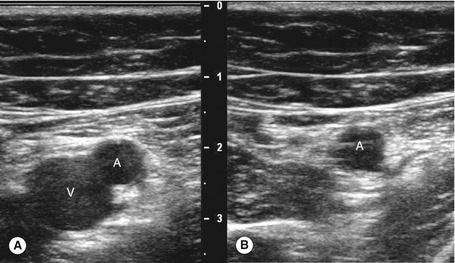
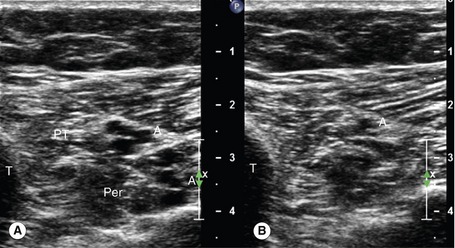
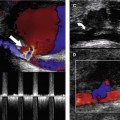
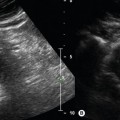
Ultrasound compression is the main method of confirming vein patency. If direct transducer pressure is applied over a vein it will collapse, as the blood pressure in the deep veins is low, unlike the pressure in the adjacent artery, and the walls will be seen to meet (coapt). The adjacent artery should demonstrate little or no distortion. In contrast, if there is thrombus in the vein it will not collapse. This technique is demonstrated in Figures 14.3 and 14.4. It should be noted that fresh thrombus, which is soft, can partially deform. Compression should be applied at frequent intervals along the length of a vein to confirm patency. Partial collapse of the vein suggests the presence of nonoccluding thrombus. In this situation, the adjacent artery may be seen to deform as the probe pressure is increased to confirm partial obstruction in the vein. Transducer compression should be applied in the transverse imaging plane rather than the longitudinal plane. This is because it is easy to slip to one side of the vein as pressure is applied in the longitudinal plane, and this may mimic compression of the vein when observed on the B-mode image. In some areas the veins lie too deep for compression to be used, such as in the pelvis and sometimes at the adductor canal or calf. Color flow imaging is useful for demonstrating patency in this situation. In order to display flow when using color flow imaging in the transverse plane, some tilting of the transducer is often necessary to obtain a Doppler angle to the vein.
The following guidelines can be used in any sequence, depending upon the areas that require assessing. It is sometimes easier to locate a specific vein by looking for the adjacent artery, especially in the calf. The reader should also refer to Chapter 9 for more details on the probe positions for imaging the calf vessels and the main vessels in the thigh and pelvis.
1. Starting at the level of the groin, the common femoral vein is imaged in transverse section and will be seen to lie medial to the common femoral artery (Fig. 14.3A; see Fig. 9.8). The common femoral vein should be compressed to demonstrate patency and is followed distally beyond the saphenofemoral junction, to the junction of the femoral vein and deep femoral vein. The proximal segment of the deep femoral vein should also be assessed for patency if possible. With the transducer turned into the longitudinal plane, the flow pattern in the common femoral vein should be assessed with color flow imaging and spectral Doppler. Flow should appear spontaneous and phasic at this level if there is no outflow obstruction. A calf squeeze can provide evidence of good flow augmentation in the proximal femoral vein, which is a useful indirect indicator of probable femoral and popliteal vein patency. Alternatively, strong foot flexion will also normally augment flow.
2. The femoral vein is then followed in transverse section along the medial aspect of the thigh to the knee, using compression to confirm patency. The vein normally lies deep to the superficial femoral artery. In the adductor canal the vein may be difficult to compress. It is sometimes helpful to place a hand behind the back of the lower thigh and push the flesh toward the transducer, which will bring the vein and artery more superficial to the transducer. Color flow imaging can also be used to confirm patency in this segment, but areas of nonoccluding thrombus could be missed. Remember that duplication of the femoral vein is relatively common, and both trunks should be examined.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree