9 Duplex assessment of lower-limb arterial disease
INTRODUCTION
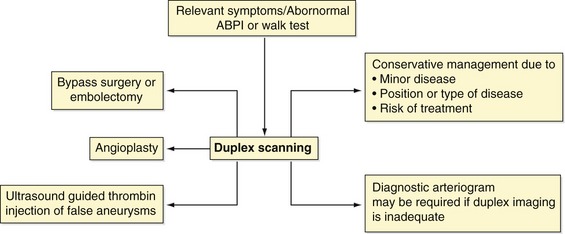
The symptoms of lower-limb arterial disease can range from mild muscle pain on exercise (claudication) to severe ischemia resulting in potential amputation. The prevalence of lower-limb arterial disease has been estimated to be in the region of 3–10%, increasing to 15–20% in persons over the age of 70 years. The disease mainly affects persons >50 years old. It was with the introduction of color flow imaging in the 1980s that duplex scanning became a practical method of assessing the lower-limb arteries. A number of studies comparing the accuracy of duplex scanning with angiography have been undertaken and published and these indicated that duplex ultrasound scanning provided comparable results to angiography (Legemate et al. 1989; Pemberton & London 1997). Although little has changed in scanning techniques over the last decade, duplex scanning continues to play a central role in the patient management pathway by allowing clinicians to formulate treatment plans without the need for diagnostic arteriograms, which, like all invasive tests, carry a small risk of complications (Egglin et al. 1995). Figure 9.1 summarizes the current role of ultrasound. It can be seen that vascular radiologists can spend less time performing diagnostic arteriograms and instead concentrate their skills on therapeutic treatment by balloon angioplasty. In some instances, the decision may be taken to treat the patent conservatively due to the location or extent of the disease, avoiding the risk of intervention. Ultrasound also has an important role to play in the acute surgical admissions unit, where acute arterial occlusions can be rapidly located and identified or other pathologies, such as false aneurysms, diagnosed. This chapter provides an overview of lower-limb arterial disorders and offers practical advice on color duplex scanning of peripheral arteries.
ANATOMY OF THE LOWER-LIMB ARTERIAL SYSTEM
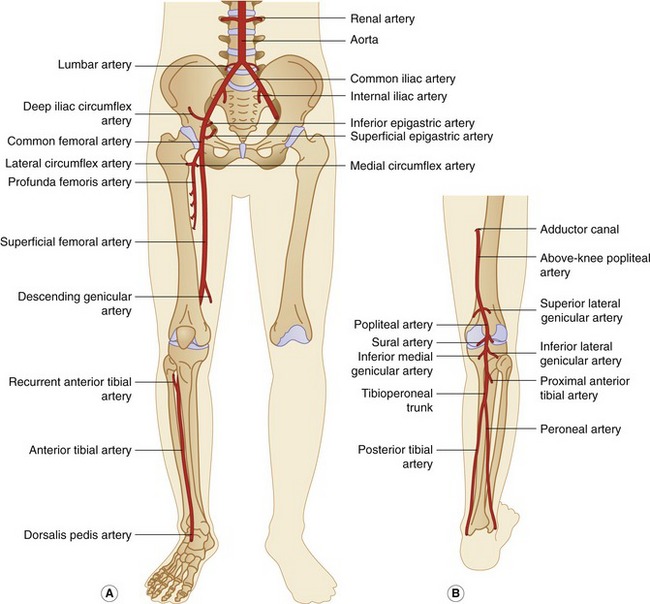
The anatomy of the lower-limb arterial system is demonstrated in Figure 9.2. The abdominal aorta has been included in this section, as it can be a source of lower-limb symptoms.
The CFA divides into the deep femoral artery, also known as the profunda femoris artery, and the superficial femoral artery (SFA) at the level of the groin. The profunda femoris artery usually runs posterolateral to the SFA and supplies blood to the thigh tissue and muscles. It also acts as an important collateral pathway in the presence of an SFA occlusion. The profunda femoris artery usually gives off the medial and lateral circumflex arteries just beyond its origin. The SFA follows a medial course down the thigh, becoming the popliteal artery at the level of the adductor canal above the knee. The SFA gives off relatively few major branches, although the descending genicular artery can act as an important collateral pathway. The popliteal artery then runs behind the knee, or popliteal fossa, and bifurcates below the knee into the anterior tibial (AT) artery and tibioperoneal trunk. The popliteal artery has a number of genicular and sural branches supplying blood to the knee joint and gastrocnemius and soleus muscles. The proximal AT artery runs in an anterolateral direction through the interosseous membrane to the anterolateral aspect of the upper calf. It then continues to run to the lower calf, becoming the dorsalis pedis artery over the dorsum of the foot. The tibioperoneal trunk can vary in length and bifurcates into the posterior tibial (PT) and peroneal arteries (Fig. 9.2B). The PT artery follows a medial course along the calf and runs behind the medial malleolus (or ankle bone) in its distal segment. The peroneal artery lies deeper than the PT artery against the border of the fibula and runs toward the lateral malleolus (outer aspect of the ankle) in its distal segment, dividing into lateral malleolar, calcaneal, and perforating branches. It is important to note that the peroneal artery is often spared in the presence of tibial artery disease. This is why its identification can be useful if a distal bypass procedure is being considered.
The forefoot is mainly supplied by the dorsalis pedis artery and the medial and lateral plantar arteries, which are terminal branches of the PT artery. The dorsalis pedis and plantar arteries anastomose to form the plantar arch, which supplies the arteries to the toes.
There are a number of anatomical variations in the lower-limb arterial system that may occasionally be encountered during routine examinations. The most common variations are listed in Table 9.1.
Table 9.1 Anatomical variations of the lower-limb arterial system
| ARTERY | VARIATION |
|---|---|
| Common femoral artery bifurcation | The bifurcation can sometimes be very high; the proximal course of the profunda femoris artery can sometimes be variable and lies posterior and medial to the superficial femoral artery in a small number of cases |
| Anterior tibial artery | High origin across the knee joint |
| Anterior tibial artery | May be small or hypoplastic |
| Peroneal artery | Origin from anterior tibial artery rather than the tibioperoneal trunk |
Collateral circulation and pathways
Although symptoms are generally proportional to the extent of disease, it is sometimes surprising to find patients with arterial occlusions but relatively minor symptoms. Conversely, some patients with one or two stenoses can experience significant impairment to their lifestyle. This variability is mainly due to the quality of the collateral circulation. If an arterial segment is severely diseased or occluded, there are often alternative pathways that are able to carry blood flow around the diseased segment, referred to as collateral vessels. In this situation, reverse flow is observed in major branches of arteries just distal to an area of severe disease, where they help to resupply blood flow to the main vessel. One such example is flow reversal observed in the internal iliac artery, supplying blood to the external iliac artery in the presence of a CIA occlusion (Fig. 9.3). It should be noted that it is very difficult to follow collateral vessels for any length using the duplex scanner, especially in the pelvis. This is not really a problem, as it is the length and severity of the disease in the main vessels that the sonographer is attempting to document. However, the quality of the collateral circulation is very important, and this can be determined by assessing the patient clinically and measuring the ankle–brachial pressure index (ABPI). Common collateral pathways are summarized in Table 9.2.
Table 9.2 Common collateral pathways of the lower-limb arteries
| DISEASED ARTERY | DISTAL NORMAL ARTERY | COMMON COLLATERAL PATHWAY |
|---|---|---|
| Common iliac artery | External iliac artery | Lumbar arteries communicating with the iliolumbar arteries of the ipsilateral internal iliac artery, which supply the external iliac artery via retrograde flow; there can also be communication between the contralateral internal iliac artery and ipsilateral internal iliac artery |
| External iliac artery | Common femoral artery | Ipsilateral internal iliac artery via pelvic connections to the deep iliac circumflex artery or inferior epigastric artery |
| Common femoral artery | Femoral bifurcation | Ipsilateral pelvic arteries filling the profunda femoris artery via the femoral circumflex arteries, which supply the superficial femoral artery via retrograde flow |
| Superficial femoral artery | Above-knee popliteal artery | Flow via profunda femoris artery (or branches of the proximal superficial femoral artery if patent) to the descending or superior genicular arteries, depending on the length of the superficial femoral artery occlusion |
| Superficial femoral artery | Below-knee popliteal artery | Profunda femoris artery branches to inferior genicular branches of the popliteal artery |
| Popliteal artery | Distal popliteal artery | Flow via the superior genicular arteries to inferior genicular arteries, depending on the level of the occlusion |
| Proximal tibial arteries | Distal tibial arteries | There are numerous arterial collateral connections in the calf, but they may not be large enough to carry sufficient flow to the foot |
SYMPTOMS OF LOWER-LIMB ARTERIAL DISEASE
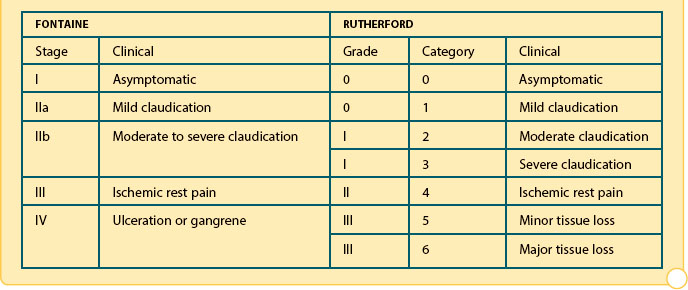
Classification of peripheral arterial disease: Fontaine’s stages and Rutherford’s categories (Rutherford et al. 1997; Reprinted from Journal of Vascular Surgery, 26/3, Rutherford et al. 1997, with permission from Elsevier)
Intermittent claudication
Atherosclerosis is a major health problem in developed countries where lifestyle factors, such as smoking and diet, can accelerate the progression of the disease. It is estimated that intermittent claudication affects approximately 4.5% of the population aged between 55 and 74 years, and there is evidence that persons with claudication have a significantly higher mortality rate from cardiac disease than nonclaudicants (Fowkes et al. 1991). Intermittent claudication is caused by arterial narrowing in the lower-limb arteries, and symptoms develop over a number of months or years. Claudication is typified by pain and cramping in the muscles of the leg while walking, which usually forces the patient to stop and rest for a few minutes in order to ease the symptoms. The severity of pain experienced and the distance a patient is able to walk can vary from day to day but, generally, walking briskly or on an incline will produce rapid onset of symptoms. The location of pain (i.e., calf, buttock, or thigh) is often associated with the distribution of disease. For instance, aortoiliac disease often produces thigh, buttock and calf claudication whereas femoropopliteal disease is associated with calf pain. There are sometimes physical signs of deteriorating blood flow in the lower limb, such as hair loss from the calf and an absence of nail growth. Claudication only occurs during exercise because, at rest, the muscle groups distal to a stenosis or occlusion remain adequately perfused with blood. However, during exercise the metabolic demand of the muscles increases rapidly, and the stenosis or occlusion will limit the amount of additional blood flow that can reach the muscles, so causing claudication.
Many patients with intermittent claudication are treated by conservative methods. This includes reduction or elimination of risk factors associated with atherosclerosis, such as smoking. Patients are also advised to undertake a controlled exercise program to build up the collateral circulation around the diseased vessel, which may ease symptoms over time. If necessary, serial ABPI measurements or exercise tests can be performed to monitor the patient’s progress. Interventional treatment is mainly by angioplasty which involves the dilation of stenoses or occlusions with percutaneous balloon catheters (see Ch. 1). Arterial stents are sometimes used to prevent restenosis, although in-stent stenosis is known to occur in a proportion of cases due to the development of intimal hyperplasia (see Fig. 9.20). Surgical bypass is usually avoided, unless the patient is suffering from severe claudication, as there is a small potential risk of complications occurring during or after surgery, which in extreme cases could lead to amputation or even death.
Chronic critical lower-limb ischemia
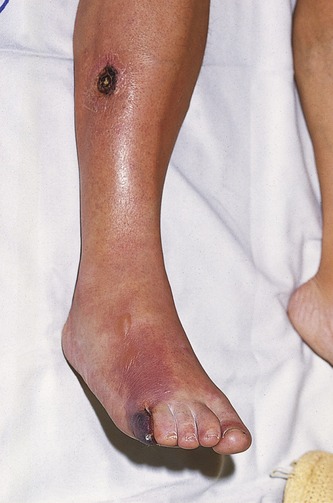
Critical lower-limb ischemia occurs when blood flow beyond an arterial stenosis or occlusion is so low that the patient experiences pain in the leg at rest because the metabolic requirements of the distal tissues cannot be maintained (Fig. 9.4). This is frequently typified by severe rest pain at night, forcing the patient to sleep in a chair or to hang the leg in a dependent position over the side of the bed. This improves blood flow due to increased hydrostatic pressure. The Trans-Atlantic Inter-Society Consensus for the management of peripheral arterial disease (TASC II) (Norgren et al. 2007) makes the following recommendation for defining critical lower-limb ischemia:
In addition the documents also states:
Acute limb ischemia
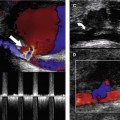
Acute limb ischemia, as the name suggests, is due to sudden arterial obstruction in the lower-limb arteries that may threaten limb viability. The position of the obstruction can be variable. Main causes of acute ischemia are listed in Box 9.1. Acute thrombosis of an existing arterial lesion, a so-called acute-on-chronic occlusion, can occur when the blood flow across a diseased segment of an artery is so slow that it spontaneously thromboses. Long segments of an artery may occlude in this situation. Acute ischemia is more likely to occur if the collateral circulation around the disease is poorly developed. An embolus may be released from other areas of the body, such as the heart or from an aneurysm, and then travels distally down the leg, eventually obstructing the artery when the arterial diameter is less than that of the embolus. An embolus frequently obstructs bifurcations such as the common femoral bifurcation or distal popliteal artery and tibioperoneal trunk. Another example is obstruction of the aortic bifurcation by an embolus projecting down both CIA origins, referred to as a saddle embolus. The body has very little time to develop collateral circulation around embolic occlusions, and the limb may be very ischemic.
BOX 9.1 Cause of acute lower-limb ischemia
• Thrombosis of an atherosclerotic artery
• Embolism from heart, aneurysm, plaque, or critical stenosis upstream (including cholesterol or atherothrombotic emboli secondary to endovascular procedures)
• Thrombosed aneurysm with or without embolization
• Thrombosis of an arterial bypass graft
• Spontaneous thrombosis associated with a hypercoagulable state
• Popliteal entrapment with thrombosis
The classic symptoms of acute ischemia are shown in Box 9.2. In this situation, emergency intervention by surgical embolectomy, bypass surgery, or thrombolysis should be performed, provided that the patient is fit enough for treatment. Left untreated, acute ischemia can lead to muscle death or necrosis. This can cause swelling of the calf muscle, and eventually the sac, or fascia, surrounding the muscles will restrict any further swelling, leading to a pressure increase within the muscle compartments. This is known as compartment syndrome, and the acute increase in intramuscular pressure can further exacerbate the muscle ischemia. If limb salvage is possible, surgical splitting of the fascia, called a fasciotomy, may be required to release the excess pressure.
DISEASE PATTERNS AND LOWER-LIMB WAVEFORM SHAPES
Caution
Remember, if the patient has just walked briskly from the waiting room to the examination table the shape of observed waveforms in normal arteries may demonstrate continuous forward flow, due to changes in peripheral resistance. The patient should be allowed to relax for several minutes before starting the examination (see Fig. 5.11B).
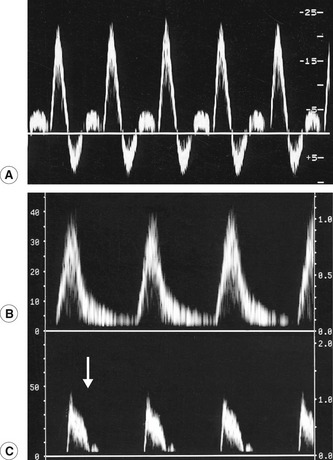
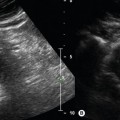
It is important to have an understanding of the different patterns of disease that may be encountered during the examination as long segments of arteries are being interrogated. Common findings include isolated or multiple stenoses, occlusions, diffuse calcified wall disease, aneurysms, and, rarely, dissections. In addition, the ability to distinguish both visually and audibly between normal and abnormal Doppler waveforms is essential (Fig. 9.5). At rest, the normal spectral Doppler display recorded from a lower-limb extremity artery, excluding the aorta, is a triphasic flow pattern with a clear spectral window (Fig. 9.5A). This characteristic pulsatile waveform shape is due to a combination of compliant distensible arterial walls and pulse wave reflections from the periphery. It may even be possible to see four phases in young healthy adults. The triphasic pattern is easily distinguished from the audio output. In elderly patients or patients with poor cardiac output, the waveform may be biphasic or even monophasic.
Waveforms with an increased systolic rise time are characteristic of disease proximal to the point of measurement (Fig. 9.5B). For example, a study by Sensier et al. (1998) demonstrated that qualitative assessment of the CFA Doppler waveform has a sensitivity of 95%, a specificity of 80%, and an accuracy of 87% for the prediction of significant aortoiliac artery disease. This study therefore suggests that observation of the CFA waveform shape is a useful technique for the investigation of inflow disease. The presence of triphasic flow with a short systolic rise time is an indicator of normal inflow. However, care should be exercised when investigating younger patients who may have a very short proximal iliac stenosis, as the arterial waveform shape may have recovered at the level of the CFA, appearing normal. Conversely, high-resistance, low-volume flow waveforms are indicative of severe disease distal to the point of measurement. One such example is the characteristic shoulder seen on the systolic downstroke of an SFA waveform recorded proximal to severe disease in the SFA (Fig. 9.5C
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree