and John P. Deveikis2
(1)
Department of Surgery, Division of Neurosurgery, and Departments of Radiology and Neurology, University of Alabama, Birmingham, AL, USA
(2)
Bayfront Medical Center, St. Petersburg, FL, USA
Abstract
Intracranial dural AV fistulas (dAVF, aka dural AV fistulas, dural arteriovenous fistulous malformation (Borden et al., J Neurosurg 82:166–179, 1995)) are acquired lesions that usually involve one of the intracranial venous sinuses. They comprise ≤10% of all intracranial vascular malformations (Al-Shahi et al., Stroke 34:1163–1169, 2003). Typically, numerous branches of the ECA, ICA, and/or vertebral artery form direct connections to a venous sinus and/or intracranial veins. Any intracranial venous sinus may be involved. Clinical features, natural history and management options depend upon the location and anatomy of the lesion. Lesions causing arterialization of intradural veins (aka retrograde leptomeningeal cortical venous flow) are classically associated with intracranial haemorrhage.
Intracranial dural AV fistulas (dAVF, aka dural AV fistulas, dural arteriovenous fistulous malformation)1 are acquired lesions that usually involve one of the intracranial venous sinuses. They comprise ≤10% of all intracranial vascular malformations.2 Typically, numerous branches of the ECA, ICA, and/or vertebral artery form direct connections to a venous sinus and/or intracranial veins. Any intracranial venous sinus may be involved. Clinical features, natural history and management options depend upon the location and anatomy of the lesion. Lesions causing arterialization of intradural veins (aka retrograde leptomeningeal cortical venous flow) are classically associated with intracranial haemorrhage. Spinal dural AV fistulas are discussed in Chap. 20.
15.1 Pathophysiology
15.1.1 Anatomy and Classification
All intracranial dAVFs consist of one or more meningeal feeding arteries that drain directly into a venous sinus or intracranial vein. Two classification systems are in common usage and both have been validated as predictive of risk of haemorrhage.3 Borden and colleagues:1
1.
Type I. Dural AVFs that drain directly into a dural venous sinus or meningeal vein.
2.
Type II. Dural AVFs that drain into a dural venous sinus with retrograde drainage into subarachnoid veins.
3.
Type III. Dural AVFs that drain directly into subarachnoid veins.
4.
Further classification into subtypes a and b indicate single or multiple fistulas, respectively.
Cognard and coworkers:4
1.
Type I. Drainage is into a sinus, with normal antegrade flow.
2.
Type II. Dural AVFs with reflux.
(a)
IIa Reflux into the sinus.
(b)
IIb Reflux into cortical veins.
(c)
IIa + b Reflux into both.
3.
Type III. Direct cortical venous drainage without venous ectasia.
4.
Type IV. Direct cortical venous drainage with venous ectasia larger than 5 mm in diameter and three times larger than the diameter of the draining vein.
5.
Type V. Drainage occurs into spinal perimedullary veins.
15.1.2 Etiology
Several lines of evidence have lead to a three-stage hypothesis for the formation of dAVFs (Fig. 15.1).5–7
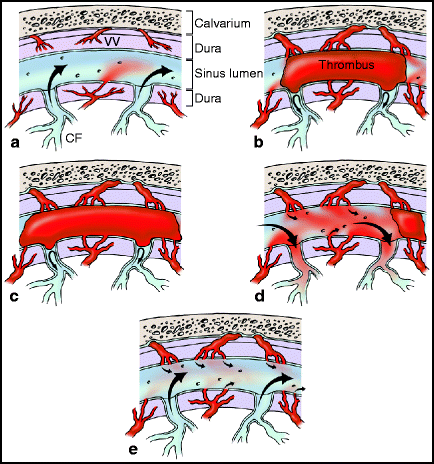

Fig. 15.1
Stages in the formation of dural arteriovenous fistulas. (a) Normal anatomy of the dural venous sinus. (b) Stage 1. Thrombosis of the venous sinus occurs, leading to diversion of flow in the entering venous tributaries. (c) Stage 2. Thrombosis of the venous sinus provides an impetus to the enlargement and expansion of nascent connections between vaso vasorum and fistulas between vaso vasorum and nearby arteries within the wall of the venous sinus. Over time, as dural arteries enlarge, grooves in the adjacent bone develop. (d) Stage 3-Partial recanalizaton. Incomplete recanalization of the thrombosed sinus occurs, (or some other venous outflow-limiting factor exists, such as venous sinus stenosis), leading to diversion of arterial flow into subarachnoid veins. (e) Stage 3-Complete recanalization. Normal venous outflow resumes, and AV fistulas may or may not continue to be patent.
1.
Stage 1. Venous sinus thrombosis is the initial event,8 possibly combined with other anatomic features that limit venous outflow, such as venous sinus stenosis.
2.
Stage 2. Nascent microscopic fistulas within the wall of the venous sinus, connecting vaso vasorum to tiny venous tributaries, enlarge. This process may be the result of a build-up of back pressure in the venous system, inflammatory changes in response to the thrombosis, and/or via an increase in angiogenic factor expression.7,9–13
3.
Stage 3. Recanalization of the thrombosed venous sinus occurs. If only partial recanalization appears, or if there is some other venous sinus outflow obstruction (like venous sinus stenosis), arterial flow is diverted into the subarachnoid venous system (i.e., retrograde leptomeningeal flow occurs).
15.1.2.1 Evidence in Favor of the Three Stage Hypothesis
1.
2.
Development of abnormal communications between dural arteries and veins appears to be an essential factor in the development of dAVFs.19
3.
4.
6.
Progressive thrombosis can transform Type I malformations into Type II malformations.
15.2 Clinical Features
2)
4)
Presentation. Symptoms and physical findings are highly variable, and depend on the location and anatomy of the lesion (see below for discussion of dAVFs by location).
5)
Significant symptoms from dAVFs are generally divided into those attributable to haemorrhage or “non-haemorrhagic neurological defects,”32,33 which are usually due to intracranial venous hypertension.
a)
Haemorrhage.
i)
In a prospective study of intracranial haemorrhage due to an intracranial vascular malformation (brain AVM, cavernoma, or dAVF), a dAVF was the underlying cause in 6.4% of cases.34
ii)
b)
Intracranial venous hypertension The intracranial venous system is valveless and so elevated pressure within arterialized venous sinuses and/or intracranial veins may be transmitted throughout the intracranial venous system. Diffuse white matter changes on MRI due to venous congestion may be seen.36,37 Decreased apparent diffusion coefficient (ADC) is observed in the brain tissue affected by retrograde cortical venous flow and is associated with brain dysfunction.38
i)
Neurological symptoms attributable to elevated intracranial venous pressure include:
15.2.1 Pulsatile Tinnitus: What Does It Mean?
Some 4% of all patients with tinnitus have pulsatile tinnitus (aka pulse-synchronous tinnitus).46 Pulsatile tinnitus is usually caused by vibrations from turbulent blood flow. Dural AV fistulas are the single most common cause of pulsatile tinnitus, followed by carotid-cavernous fistulas and atherosclerotic carotid stenosis.47 In a series of 24 patients with Borden Type I dAVFs of the transverse-sigmoid region, pulsatile tinnitus was the presenting symptom in all patients.27 A vascular disorder is present in 42% of patients with pulsatile tinnitus; a non-vascular disorder is the cause of pulsatile tinnitus in 14% of patients.47
1.
Vascular causes
(a)
dAVF
(b)
ICA stenosis or occlusion
(c)
Carotid dissection
(d)
Fibromuscular dysplasia
(e)
Persistent stapedial artery
(f)
Aberrant or lateralized ICA
(g)
Intracranial venous hypertension
(j)
Turbulent flow through a dominant internal jugular vein
(k)
Vertebral artery-venous fistula
2.
Non-vascular causes of pulsatile tinnitus
(a)
Middle ear disorders (otitis media, glomus tympanicum, cholesteatoma)
(b)
Labyrinth disorders (otospongiosis)
(c)
High cardiac output disorders (anemia, thyrotoxicosis, valvular heart disease)
(d)
Skull base tumors
3.
Work-up for pulsatile tinnitus
(a)
Pulsatile tinnitus is either objective (a bruit on the skull or mastoid process can be heard by the examiner) or subjective (only the patient can perceive it). Objective tinnitus should raise a high suspicion of an underlying vascular abnormality and a catheter angiogram is frequently indicated.
(b)
Imaging
CT is insensitive in the assessment of pulsatile tinnitus
MRI is more sensitive than CT
In patients with subjective pulsatile tinnitus, MRI/MRA defined anatomical abnormalities that may contribute to pulsatile tinnitus in 63% of patients.50
(c)
In the absence of objective pulsatile tinnitus, MRI/MRA is an appropriate initial diagnostic step.50
15.2.2 Imaging
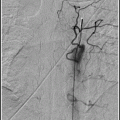
15.2.2.1 Angiography
Catheter angiography is the best technique for the diagnosis of an intracranial dAVF.51,52
1.
Technique
(a)
A complete 6-vessel cerebral angiogram is necessary, as some lesions may have feeding arteries from both sides, as well as from the ICA or vertebral arteries as well as the ECAs. For example, it is not uncommon for a transverse/sigmoid sinus fistula to receive feeders from both middle meningeal arteries.
(b)
Each angiogram should be carried well into the venous phase, to assess intracranial vein and venous sinus anatomy.
2.
Pertinent findings
(a)
The single most important goal of angiography in patients with a dAVF is to look for retrograde leptomeningeal venous drainage.
Good visualization of the venous phase is essential.
Tortuous, engorged veins seen during the venous phase is termed pseudophlebitic pattern and is a sign of venous congestion.53 A pseudophlebitic pattern was seen in 81% with retrograde leptomeningeal venous drainage and in only 8% of dAVF patients with drainage into a venous sinus only.53
Subtle findings seen during the venous phase include drainage via pial or medullary collateral veins, focal regions of delayed circulation and venous rerouting to the orbit or to transosseous veins.53
(b)
The arterial feeders must be characterized.
Occasionally, a dAVF may have only a single (or two or three) endovascularly accessible arterial pedicles and thus be a prospect for curative arterial embolization.
(c)
Venous outflow obstruction should be checked for (e.g., venous sinus stenosis or thrombosis).
15.2.2.2 MRI
MRI may not show some dAVFs and should not be used instead of catheter angiography to exclude the presence of a dAVF. Remember: a normal MRI does not exclude the presence of a dAVF.
1.
MRI findings in patients with dAVFs:
2.
MRI may provide complementary information that is not available with catheter angiography:
(a)
MRI evidence of white matter edema (diffuse T2 hyperintensity in the white matter) is evidence of venous congestion.36
(b)
Evaluation for hydrocephalus.
(c)
Quantitative assessment of cerebral blood volume by dynamic susceptibility contrast MRI can provide quantitative information about retrograde cortical venous drainage.55
(d)
Decreased apparent diffusion coefficient (ADC) can be seen with retrograde cortical venous flow and is associated with brain dysfunction.38
15.2.3 Natural History
Intracranial dAVFs are dynamic. In a study of 112 patients with dAVFs who were managed conservatively, 12.5% showed spontaneous occlusion of the fistula (most commonly transverse and cavernous sinus locations) and 4.0% showed conversion to a higher grade dAVF.58
15.2.3.1 Risk of Haemorrhage
The overall annual risk of haemorrhage in patients with intracranial dAVFs is 1.8%, with a case fatality rate of 20% with haemorrhage.31 However, the natural history of dAVFs depends strongly on the pattern of venous drainage. Most Borden Type I lesions (aka Cognard Type I or IIa) are “benign” whereas higher grade lesions are “aggressive.”
15.2.3.2 Borden Type I dAVFs: Most Are Benign
1.
In a series of 112 patients with Borden Type I lesions followed for median time of 27.9 months, observation and/or palliative therapy resulted in a benign and tolerable level of disease in 98.2% of cases.26
(a)
Palliation consisted of embolization to reduce symptoms without obliteration of the lesion.
(b)
In 2% of cases conversion to a higher grade lesion occurred, with progressive thrombosis of venous outlets.
2.
A previous study reported on a group of 54 patients with Borden Type I lesions.59 During a mean follow-up period of 33 months, 53 (98%) patients had good outcomes (i.e., symptoms were resolved, improved, or unchanged). One death was reported; this patient had a complex torcular dAVF and his death was attributed to venous hypertension and elevated intracranial pressure.
3.
Cognard and colleagues reported on a group of seven patients with Type I lesions, followed for a mean time of 7 years, all of whom developed a worsening in both the venous drainage pattern and clinical symptoms.60 The authors did not indicate the number of patients with Type I lesions in the entire group, so an incidence of worsening cannot be calculated from this paper.
15.2.3.3 Borden Type II and III dAVFs: Aggressive
Patients with high grade dAVFs are at significant risk of haemorrhage or neurological problems because of intracranial venous hypertension.
1.
Risk of haemorrhage or non-haemorrhagic neurological deficit.
(a)
van Dijk and colleagues reported on 20 patients with Borden Type II or III lesions who were either partially treated or not treated at all and followed for a mean period of 4.3 years.33
The annual rate of haemorrhage was 8.1% and the annual rate of a non-haemorrhagic neurological deficit was 6.9%, yielding an annual event rate of 15%.
(b)
Davies and coworkers reported on 14 patients with Borden Type II or III lesions who were followed for a mean period of 25 months without or prior treatment.32
The annual rate of haemorrhage was 19.2% and the annual rate of a non-haemorrhagic neurological deficit was 10.9%. The overall annual mortality rate was 19.3%.
2.
Risk of rehaemorrhage.
(a)
In a series of patients with dAVFs with cortical venous drainage presenting with haemorrhage, rebleeding occurred in 35% within 2 weeks of the initial haemorrhage.61
15.3 Management
Management options for patients with a dAVF:
1.
Conservative management
2.
Endovascular techniques
3.
Surgery
4.
Radiosurgery
5.
A combination of endovascular treatment, radiosurgery, and/or surgery
15.3.1 Conservative Management
Conservative management (i.e., no endovascular or surgical procedure, with or without surveillance imaging) is reasonable in certain situations. Asymptomatic or minimally symptomatic Borden Type I lesions without evidence of cortical venous drainage may be managed expectantly. It is well established that spontaneous regression of dAVFs occurs in some cases. This is particularly true of cavernous sinus dAVFs,62,63 in which spontaneous regression has been reported in up to 73% of cases.64–66 Intermittent manual compression is also effective for some patients with cavernous sinus or transverse-sigmoid lesions.67,68
15.3.2 Endovascular Treatment
Generally the most effective treatment of dAVFs is occlusion of the draining vein.7 In most cases, obliteration of the lesion can only be accomplished by treatment of the venous side of the lesion. Transvenous techniques appear to carry the highest success rates among endovascular techniques. Even successful arterial embolization usually occurs only when the microcatheter is positioned well within or adjacent to the nidus, so that embolic material can be pushed through the nidus into the venous side. If only feeding arteries are occluded and not the draining vein, collateral vessels usually develop and the fistula will recur. Conversely, it is equally important to ensure that normal venous drainage is preserved after embolization to avoid exacerbated venous hypertension and risk of haemorrhage.
Embolization of the arterial feeders alone is usually only palliative. Embolization of feeders proximal to the nidus will nearly invariably be followed by recruitment of a new arterial supply and possibly the redirection of the venous outflow with an increased risk of haemorrhage.
15.3.3 Surgery
Surgery for intracranial dAVFs has evolved considerably over the last three decades from simple ligature of feeding vessels (which produced success rates of only 0–8%),69 to blood-soaked fistula resection and packing of the venous sinus,70 or to more elegant interruption of the draining vein when the anatomy is favorable.71 Although endovascular techniques are presently first-line treatments for many dAVFs, surgery remains standard for anterior fossa dAVFs.69 A variety of hybrid surgical/endovascular procedures have evolved as well, including surgical exposure of the superior ophthalmic vein for embolization of the cavernous sinus72,73 and craniectomy with direct puncture of a venous sinus.74
15.3.4 Radiosurgery
Preliminary reports of radiosurgery for intracranial dAVFs are encouraging.75,76 In many cases embolization is combined with radiosurgery.77 Overall rates of lesion obliteration are 58–83%.76,78–81 Interestingly, a single report indicated a higher obliteration rate with embolization followed by radiosurgery (83%) compared to radiosurgery alone (67%),81 which is the opposite of the situation with radiosurgery for brain AVMs.82–84
15.4 Dural AVFs by Location
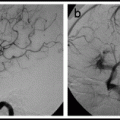
In North American and European series, the transverse-sigmoid sinus region is the most common location for dAVFs;31,35 in a Korean report, the cavernous sinus was the most common location, accounting for 64% of cases (Fig. 15.2).85
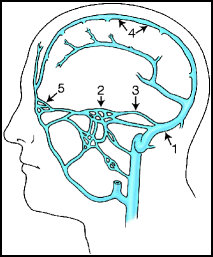

Fig. 15.2
Most common locations of dural arteriovenous fistulas.
Most common locations for dAVFs are.86
1.
Transverse-sigmoid sinus 35%
2.
Cavernous sinus 35%
3.
Tentorium/Superior petrosal sinus 5%
4.
Superior sagittal sinus 5%
5.
Anterior fossa 5%
15.4.1 Transverse-Sigmoid Sinus dAVFs
The transverse-sigmoid sinus (aka lateral sinus) is the most common location for intracranial dAVFs, comprising 38% of all intracranial dAVFs.86
15.4.1.1 Clinical Features
2.
Borden Type I dAVFs of the transverse-sigmoid sinus (analysis of 24 cases):27
(a)
Seventy-nine percent women; median age at onset of symptoms: 54 years.
(b)
Unilateral pulsatile tinnitus was the presenting symptom (and a bruit could be heard) in all patients.
(c)
MRI was normal in all patients.
4.
Anatomy
(a)
Feeders – may be bilateral
ECA branches are most common: occipital, posterior auricular, ascending pharyngeal, middle meningeal, accessory meningeal and superficial temporal arteries.
ICA branches: meningohypophyseal trunk, inferolateral trunk.
Vertebral artery branches: posterior meningeal artery, cerebellar falcine and muscular branches.
(b)
Venous drainage
The transverse or sigmoid sinus is stenotic or occluded in a significant percentage of cases.5
Retrograde venous flow, when present, may be into occipital and parietal cortical veins.
5.
Presentation
(a)
Haemorrhage or intracranial venous hypertension
Patients with transverse-sigmoid dAVFs present with haemorrhage or other serious symptoms in only 11% of cases.51
15.4.1.2 Management
Relative to dAVFs in other locations, theses lesions are often benign and frequently require treatment only to alleviate symptoms such as pulsatile tinnitus. Lesions with aggressive characteristics, such as retrograde venous flow and/or venous hypertension, should be treated.
Selection of the treatment strategy involves weighing several different factors. Paramount among these is the venous anatomy; other considerations include the clinical gravity of the situation, the arterial anatomy and the patient’s ability to undergo endovascular and/or surgical procedures. A systematic review in 1997 found that combined therapy (endovascular plus surgical treatment) was significantly more effective than either therapy alone (P < 0.01).69
1.
Manual compression
2.
3.
Embolization
(a)
Venous embolization
Venous embolization, or venous embolization combined with arterial embolization, results in significantly higher cure rates compared to arterial embolization alone. Successful venous embolization depends upon identifying patients with favorable anatomy for this approach; occlusion of a sinus that freely communicates with normal venous structures can cause a venous infarction and haemorrhage.
Suitable venous anatomy:
The affected sinus is compromised and no longer contributes to the drainage of normal tissue.1,91,92
Parallel venous channel. Caragine and colleagues reported on ten patients with dAVF consisting of arterial feeders converging on a “parallel venous channel,” that was separate from, but in communication with the transverse or sigmoid sinus.93 Embolization of the venous channel and cure of the fistula, with preservation of the venous sinuses, was achieved in all patients.
Venous approaches
Femoral venous access is the most commonly reported route; alternatives include direct puncture of the internal jugular vein and craniectomy with direct puncture of a venous sinus.74
(b)
Arterial embolization
Arterial embolization is rarely curative and should be reserved for palliation or as an adjunct to venous embolization.94 or surgery.
(c)
Embolization results
Most published series of transverse-sigmoid dAVF embolizations employed a combination venous/arterial strategy in some patients and venous embolization only in others.
4.
Venous sinus angioplasty and stenting
(a)
Treatment of a transverse sinus dAVF by sinus recanalization, angioplasty and stent placement has been reported.97
15.4.2 Cavernous Sinus dAVFs
Carotid-cavernous fistulas (CC fistulas) may be direct or indirect. Direct CC fistulas (aka high flow CCFs) consist of a defect in the wall of the ICA, causing a shunt between the ICA and the cavernous sinus (e.g., traumatic CCF or ruptured cavernous segment aneurysm). Indirect CC fistulas (aka low flow CC fistulas) are equivalent to dAVFs of the cavernous sinus; they comprise about 35% of all dAVFs.86 Barrow and colleagues established the following classification scheme:65
1.
Type A. Direct shunt between ICA and cavernous sinus (e.g., traumatic CCF or ruptured cavernous segment aneurysm). Direct CC fistulas are discussed separately in the Appendix.
2.
Type B. Indirect fistula between branches of the ICA and the cavernous sinus.
3.
Type C. Indirect fistula between branches of the ECA and the cavernous sinus.
4.
Type D. Indirect fistula between branches of both the ICA and ECA and the cavernous sinus.
15.4.2.1 Indirect CC Fistulas: Clinical Features
1.
Most patients are women in the sixth or seventh decade of life.
4.
Anatomy
(a)
Feeders
May be bilateral.
ECA: Branches of the internal maxillary, middle meningeal, accessory meningeal, ascending pharyngeal.
ICA: Cavernous segment branches.
(b)
Venous drainage
Highly variable.
Impaired venous drainage is typical and enlargement of the superior ophthalmic vein is a frequent finding.
Cortical venous drainage is present in 31–34% of cases.100
5.
Presentation
(b)
(d)
So-called “white-eye” CC fistulas occur when posterior venous drainage predominates and a painful ocular motor nerve palsy develops without congestive orbital features.104
6.
Imaging
(a)
CT: Typical findings include proptosis and superior ophthalmic vein enlargement.
(b)
MRA: May show an enlarged superior ophthalmic vein.
(c)
A catheter angiogram remains the gold standard for a complete evaluation of an indirect CC fistula.105 Angiography is most important for assessing the presence of retrograde cortical venous flow, as well as delineating feeders and the precise pattern of venous drainage; all of these critical anatomic features of any given CC fistula are poorly seen with noninvasive imaging.
15.4.2.2 Indirect CC Fistulas: Management
Spontaneous resolution of an indirect fistula is not uncommon, with reported rates of spontaneous remission ranging from 5.6%63 to 73%.64–66 Some authors, like the senior author of this handbook, prefer to attempt conservative management of most patients with indirect CC fistulas, whereas the junior author offers intervention to most patients with bothersome symptoms. Barrow and colleagues proposed the following indications for treatment:65 (1) Visual deterioration; (2) Obtrusive diplopia; (3) Intolerable bruit or headache; and (4) “Malignant” proptosis with untreatable corneal exposure. The presence of retrograde cortical venous drainage is also a good indication for treatment.
Selection of technique: Venous occlusion is the most reliable method to treat indirect CC fistulas. A systematic review in 1997 found overall success rates of 78% for transvenous approaches and 62% for transarterial approaches.69
1.
Manual compression
(a)
Technique:67 Instruct the patient to use the opposite hand to locate the pulse of the carotid artery in the mid-neck region just lateral to the trachea. Gradually increasing pressure is applied until the palpable pulse is stopped. The opposite hand is used in case hemispheric ischemia develops. Compression is maintained for 10–15 s at a time, 2–3 times an hour.
Contraindications: Cervical carotid artery disease (atherosclerosis, dissection), sick sinus syndrome, poor patient compliance.
(b)
Results: In 30% of cases closure occurred within a mean of 41 days (range, several minutes to 6 months).67
2.
Embolization
(a)
Venous: Most effective technique. Several different routes to the cavernous sinus may be used; the most commonly used are the inferior petrosal sinus and the superior ophthalmic vein.
(b)
Arterial: Embolization of arterial feeders is rarely curative and should be used only for palliation in select cases.
(c)
(d)
Endovascular results. Overall results in two large recent series are very favorable:
Complete cure: 90–94.5% of cases.100,107
Fistula closure will usually result in normalization of intraocular pressure.121 Improvement in vision may take longer and is less certain, as several different mechanisms may contribute to vision loss (e.g., optic neuropathy, keritis and corneal ulceration, vitreous haemorrhage, retinal ischemia, or retinal detachment).
3.
Radiosurgery
(a)
Several reports have been published on the use of radiosurgery for the treatment of cavernous CC fistulas.75,78,122,123 Although the results are generally favorable, with obliteration rates >80%, the wide variety of techniques used (dosing, use of embolization, lesion anatomy) make it difficult to make firm conclusions about the usefulness of radiosurgery in this setting.
15.4.3 Tentorial dAVFs
Tentorial dAVFs (aka superior petrosal dAVFs)101,124 comprise about 5% of all intracranial dAVFs.86 Although in most cases, the lesion is located on the petrous ridge and involves the superior petrosal sinus, most publications refer to them as “tentorial” dAVFs.71,125–127 These lesions are thought to be prone to haemorrhage and are difficult to treat by surgery and by embolization. All, or nearly all, tentorial dAVFs are Borden Type II or III lesions.32,125 Picard and colleagues classified tentorial dAVFs:128
1.
Tentorial marginal type, located along the free edge of the tentorium.
2.
Tentorial lateral type, located adjacent to the lateral venous sinuses.
3.
Tentorial medial type, located adjacent to the straight sinus and torcula.
15.4.3.1 Clinical Features
1.
Anatomy
(a)
(b)
Venous drainage
Drainage is retrograde in all cases.127
Cerebral and/or cerebellar veins
Basal vein of Rosenthal
Pontine and paramesencephalic veins
Cervical paramedullary spinal veins
2.
3.
Imaging.127
(a)
CT: Haemorrhage centered in the ambient cistern or posterior fossa
(b)
MRI: High signal on T2-weighted images indicating edema in the thalamus, midbrain, and cerebellum
15.4.3.2 Management
Tentorial dAVFs are aggressive lesions and should be obliterated when feasible. Incomplete treatment should be avoided, as recruitment of new feeders and redirection of venous outflow may occur.136 No single treatment strategy is ideal; selection of surgery and/or embolization depends on the clinical situation (vascular anatomy, patient age and health status, etc.). Because of the aggressive nature of these lesions, late (1–2 years post-treatment) follow-up catheter angiography should be done, even in cases in which an angiographic cure is obtained.
1.
Get Clinical Tree app for offline access

Surgery
(a)
Surgery is most effective for patients who are good candidates for a craniotomy and who have a lesion with a single, surgically accessible draining vein.
(b)




Technique: Surgical technique for tentorial dAVFs has evolved considerably in recent years.71,125,126 In most cases, resection of the nidus is not necessary; obliteration of the lesion can be accomplished by coagulation and division of the arterialized draining vein or veins, when the anatomy is suitable.71,126 Interruption of the draining vein may be effective when no other pathway for venous drainage exists.71 Lewis and coworkers, however, emphasized the importance of surgical interruption of the arterial supply, rather than the draining veins.129 The route of cranial access (e.g., suboccipital versus subtemporal craniotomy) depends on where the arterialized veins are located. Pre-operative arterial embolization may facilitate surgery.125,127
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree