CHAPTER 2 Dynamic Functional and Physiological Techniques
PHYSICAL PRINCIPLES
Diffusion-Weighted Imaging
Diffusion is defined as the process of random molecular thermal motion occurring at a microscopic scale. Diffusion of water in biologic systems, particularly within the brain, is affected not only by the complex interaction between the intracellular and extracellular compartments but also by the cytoarchitecture of the microstructures and permeability barriers. Diffusion of water molecules through the magnetic field gradient produces intravoxel dephasing and a loss of signal intensity. Because this microscopic diffusional motion is so small, a large gradient strength and/or duration is needed to produce observable signal loss from diffusion. By utilizing bipolar pulsed gradient methods, microscopic diffusional motion is detected by change in the magnitude of moving spins due to phase dispersion. To detect this highly sensitive motion, ultrafast imaging, such as the echoplanar imaging (EPI) technique, is needed to acquire a sufficient number of images in the range of milliseconds to produce meaningful information.1
The apparent diffusion coefficient (ADC) characterizes the rate of diffusional motion (given in millimeters squared per second). The ADC takes into consideration the heterogeneous environment of brain cytoarchitecture and factors other than diffusion, such as temperature, perfusion, and metabolic rates that can affect the measurement of microscopic thermal motion. High ADC implies relatively unrestricted water motion. Low ADC indicates restricted diffusional motion, as seen in acute cerebral ischemia. The diffusion sensitivity parameter, b value, is related to duration, strength, and time interval between the diffusion-sensitizing gradients. A typical b value used in clinical imaging is in the range of 900 to 1000 s/mm2. The higher the b value, the more sensitive the diffusion imaging is for obtaining greater contrast and detecting areas of restricted water motion.2
Anisotropic diffusion is defined as having different diffusional motion in different directions, as is the case in normal myelinated white matter tracts in the brain. Diffusion of water molecules is far less restricted along the parallel plane of the axonal fibers than in perpendicular directions. White matter anisotropy can be demonstrated by comparing diffusion-weighted images with bipolar gradients placed in three orthogonal directions. By combining the information from the three orthogonal data sets, an orientation-independent image is created without the artifact from normal white matter anisotropy.3
EPI is currently the most widely used MRI technique for clinical application of diffusion-weighted imaging (DWI) for the diagnosis of acute stroke and other brain disorders such as abscess, epidermoid, traumatic shearing injury, or necrotic encephalitis. EPI is the fastest available MRI method. It allows the entire set of echoes needed to form an image to be collected within a single acquisition period of 25 to 100 ms.4 The data are obtained by forming a train of gradient echoes by repeated reversal of a large gradient capable of very rapid polarity inversion to complete k-space filling after a single radiofrequency pulse. Each gradient echo is phase encoded separately by a very brief blipped gradient or a weak constant phase-encoding gradient. Although the long echo train renders the images sensitive to chemical shift and magnetic susceptibility artifacts, EPI virtually eliminates motion artifact. The chemical shift artifact is overcome by routine use of lipid suppression, whereas the magnetic susceptibility artifact is manifested prominently at air/bone/tissue interfaces such as those at the skull base, paranasal sinuses, orbits, and petrous temporal bone.5–7
Perfusion-Weighted MRI
Currently available perfusion-weighted imaging methods in clinical practice consist of arterial spin labeling (ASL), dynamic contrast-enhanced (DCE) MRI, and dynamic susceptibility-weighted contrast-enhanced (DSC) MRI. All three methods provide some type of a quantitative measurement of cerebral hemodynamic variables, such as cerebral blood flow (CBF), cerebral blood volume (CBV), and capillary permeability. Unlike DCE or DSC MRI, ASL is unique in that it does not require administration of exogenous contrast agent and uses tagged arterial blood spin as a source of endogenous contrast agent to measure CBF. A recent study has shown a promising role of ASL-derived CBF measurement as a complementary hemodynamic variable to more widely used DSC-derived CBV measurements in patients with glioblastoma multiforme.8 DCE MRI proposes to quantify the steady-state exchange of MRI contrast agent, gadolinium (Gd-DTPA), between the intravascular and the interstitial tissue compartment and has emerged as a promising method for diagnosis and prognosis of glioblastoma multiforme.9,10 Ktrans, also known as a volume transfer constant, is the most widely used quantitative DCE MRI variable and reflects the rate of transfer of Gd-DTPA across the endothelial membrane. Ktrans reflects the leakiness of tumor vasculature and has been used to grade gliomas.11,12 Several recently published reports suggest that Ktrans is capable of detecting the direct vascular effect of antiangiogenic therapy and thus is a promising candidate as a quantitative, clinically valid, endpoint for clinical trials.13,14 Whereas DCE MRI measures Gd-DTPA in a steady-state, DSC MRI exploits the first-pass transit of Gd-DTPA within the intravascular compartment. Its most widely used hemodynamic variable, CBV, proposes to measure bulk vessel density.15 DSC-derived CBV measurements have been extensively used to grade gliomas,16,17 evaluate tumor vasculature,18,19 differentiate recurrent tumor from treatment effect,20 and assess prognosis of patients with glioma.21 Other hemodynamic variables derived from DSC MRI such as the peak height and the percentage of signal recovery have shown their roles in further characterizing spatial heterogeneity of tumor vasculature22 and in differentiating glioblastoma multiforme and single brain metastasis by virtue of fundamental difference in leakiness of tumor vessels between the two tumor types.23
Dynamic Susceptibility-Weighted MRI (DSC MRI)
DSC MRI is a fast, contrast-enhanced, EPI-based technique that exploits the first-pass effect of intravenous contrast agent within the intravascular compartment of the cerebrovascular system. When a paramagnetic agent such as Gd-DTPA passes through the cerebrovascular system, it produces T2* signal loss due to its local magnetic susceptibility. By exploiting the intravascular compartmentalization of Gd-DTPA and the resultant susceptibility effect, an indirect measure of bulk vessel density and hence CBV can be derived from the susceptibility signal intensity time curve. The passage of Gd-DTPA causes changes in both T2 and T2* so that both spin-echo and gradient-echo EPI sequences provide robust measurements of CBV. Gradient-echo sequences are, however, much more sensitive. When a paramagnetic contrast agent such as Gd-DTPA passes through the cerebrovascular system it induces differences in local magnetic susceptibility between vessels and the surrounding tissue. Although the vascular space is a small fraction of the total tissue blood volume (4%-5%), this compartmentalization of contrast agent causes targeted paramagnetism within the intravascular spins as well as the surrounding spins within a given voxel. Thus, both intravascular and extravascular spins experience a reduction of T2* that leads to a large transient signal loss of approximately 25% in normal white matter with a standard dose of contrast (0.1 mmol/kg). T2W spin-echo images are less sensitive and require double or even quadruple contrast agent doses to give substantial signal changes during the bolus passage. On the other hand, gradient-echo sequences are more prone to magnetic susceptibility artifacts. Asymmetric spin-echo EPI sequences provide a potentially useful compromise between gradient-echo and spin-echo EPI. In asymmetric spin-echo EPI sequences the echo center is displaced from the Hahn echo time, giving a mixture of T2 and T2* weighting. The degree of asymmetry can be adjusted to trade off sensitivity against susceptibility to artifacts.20,21 Thus, when imaging lesions near brain/bone/air interfaces, such as the temporal or inferior frontal lobes where these artifacts are more pronounced, spin-echo sequences may be preferable. However, artifacts in gradient-echo images can be overcome to a large extent by reducing the slice thickness.22 Although this reduces signal-to-noise ratio, we have found that this technique still provides diagnostic images. A second advantage of spin-echo sequences is that simulations and phantom experiments suggest spin-echo images will only be sensitive to contrast agent within the capillaries whereas gradient-echo sequences will be sensitive to contrast in both capillaries and larger vessels.23 Although contamination by venous signals in gradient-echo images will potentially cause overestimates of CBV, it is relatively easy to identify the location of veins and make measurements of CBV in regions of interest that avoid them.
Proton MR Spectroscopy (MRS)
Magnetic resonance spectroscopy (MRS), the physical principle of which has been around since the 1940s, provides a measure of biochemical changes in the brain.24 A small change in the Larmor resonance frequency of a nucleus (i.e., chemical shift) generated by circulating electrons surrounding the nuclei interacting with the main magnetic field can be measured and displayed as spectral format to detect alterations in chemical composition of brain.25 The most common nuclei that are used are 1H (proton), 23Na (sodium), and 31P (phosphorus). Proton spectroscopy (1H MRS) is easier to perform and provides much higher signal-to-noise ratio than either sodium or phosphorus. For the scope of this textbook, only proton spectroscopy will be discussed.
1H MRS can be performed within 10 to 15 minutes and can be added on to conventional MRI protocols. It can be used to serially monitor biochemical changes in tumors, stroke, epilepsy, metabolic disorders, infections, and neurodegenerative diseases. In the brain, several metabolites can be measured using 1H MRS (Table 2-1). Each metabolite appears at a specific parts per million (ppm), and each reflects specific cellular and biochemical processes. In normal brain, different regions can have different chemical composition and hence variable amounts of each metabolite. Normal gray matter tends to have higher levels of choline than does white matter. N-acetyl-aspartate (NAA) is a neuronal marker and decreases with any process that compromises neuronal integrity. It can be markedly elevated in Canavan disease, a rare genetic leukodystrophy in which there is lack of an enzyme aspartoacylase, leading to abnormal accumulation of NAA. Choline is elevated in any disease that results in cellular membrane turnover, such as tumor or inflammatory process. Creatine reflects a measure of energetics in the brain. Lactate provides a measure of anaerobic metabolism and hypoxic condition. Lipid reflects an end product of tissue destruction and necrosis.26 Myoinositol is considered to be an astrocyte marker and can be elevated in Alzheimer’s disease.27
TABLE 2-1 Proton MRS Metabolites
| Parts Per Million | Metabolite | Biologic Correlate |
|---|---|---|
| 0.9–1.4 | Lipids | Tissue necrosis or destruction |
| 1.3 | Lactate | Anaerobic glycolysis |
| 2.0 | N-acetyl-aspartate (NAA) | Neuronal marker |
| 2.2–2.4 | Glutamine/GABA | Neurotransmitter |
| 3.0 | Creatine | Energy metabolism |
| 3.2 | Choline | Cell membrane turnover |
| 3.5 | Myoinositol | Glial/astrocyte marker |
IMAGING
Parameters/Protocol
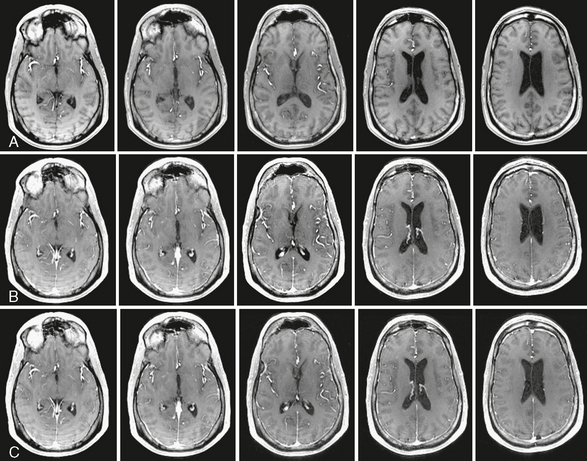
Table 2-2 lists the most widely accepted imaging parameters/protocol for DWI, three types of perfusion-weighted imaging, and proton MRS.
For the three different types of perfusion-weighted MR imaging, each requires specific imaging parameters, as listed in Table 2-2.
Proton MR spectroscopic (1H MRS) imaging methods vary depending on the spatial coverage (single vs. multiple voxel), thickness (2D vs. 3D), and echo times (short, medium, and long) used.
Normal Appearance of Images by Technique
Diffusion-Weighted Imaging
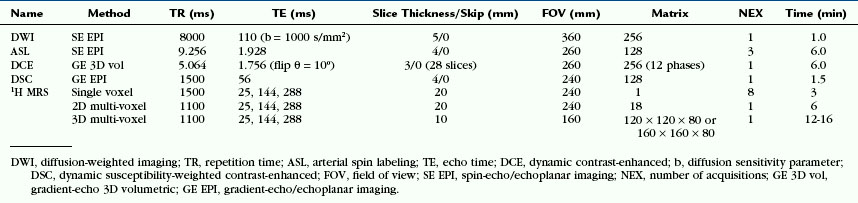
In normal brain, there should not be any areas of reduced diffusion on DWI. The cerebrospinal fluid (CSF) within the ventricles has the lowest signal because the protons in CSF have the least restriction of motion, and normal white matter with highly organized axonal tracts such as the corpus callosum has the highest signal, as shown in Figure 2-1.
Perfusion-Weighted Imaging
Arterial Spin Labeling
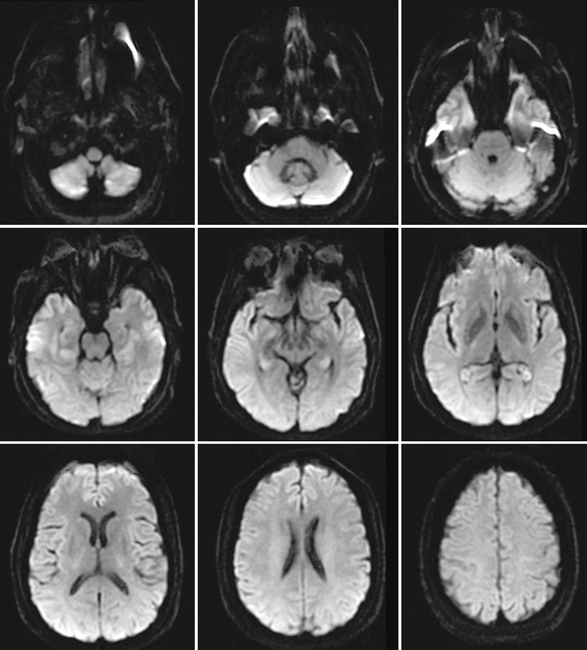
In normal adult brain the cerebral blood flow to gray matter is approximately two to three times greater than that of white matter. In normal pediatric brain there is usually an increased signal-to-noise ratio as well as globally elevated absolute CBF when compared with adults. This globally increased signal intensity within normal pediatric brain has been attributed to higher baseline CBF, faster mean transit time, increased baseline magnetization values in gray and white matter, and increased T1 values in blood and tissue. ASL images of normal adult brain are shown in Figure 2-2.
Dynamic Contrast-Enhanced MRI
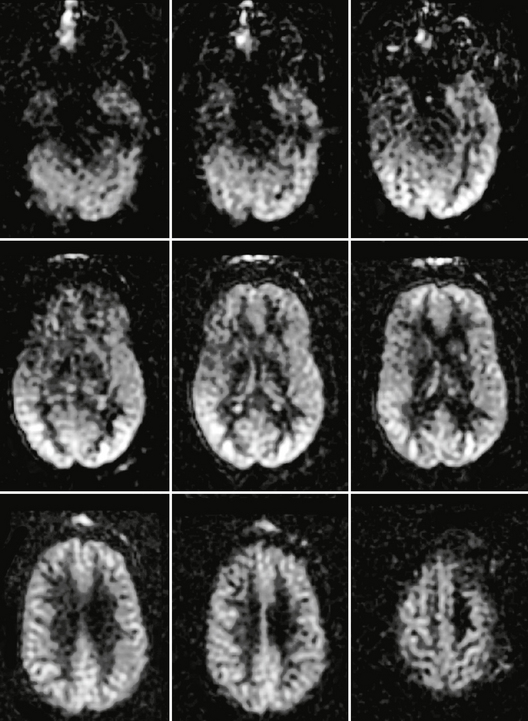
In normal brain with intact blood-brain barrier, the degree of leakage across the blood vessel is negligible. Therefore, DCE MRI of normal brain shows minimal enhancement, hence leakage of gadolinium contrast agent, whereas blood vessels are intensely enhancing. DCE images of normal adult brain are shown in Figure 2-3.