Outline
Epidemiology, 967
Risk Factors, 967
Pathophysiology, 968
Clinical Presentation, 969
Diagnostic Tests, 969
Sonographic Diagnosis of Ectopic Pregnancy, 971
Ectopic Pregnancies in Unusual Locations, 978
Interstitial Ectopic Pregnancy, 978
Rudimentary Horn Ectopic Pregnancy, 985
Cervical Ectopic Pregnancy, 986
Myometrial/Cesarean Scar Ectopic Pregnancy, 990
Ovarian Ectopic Pregnancy, 991
Abdominal Ectopic Pregnancy, 991
Retroperitoneal Ectopic Pregnancy, 993
Chronic Ectopic Pregnancy, 995
Posthysterectomy Ectopic Pregnancy, 996
Heterotopic Pregnancies, 996
Management and Treatment Options, 996
Conclusion, 998
Summary of Key Points
- •
The incidence of ectopic pregnancy (EP) in the United States is increasing as a result of an increase in the number of patients with risk factors as well as an increase in diagnosis due to earlier presentation and detection.
- •
Mortality rate from EP is decreasing because of improved diagnostic techniques and heightened awareness among clinicians and patients.
- •
The most common predisposing risk factor for EP is tubal abnormality. However, up to 50% of patients with EP have no known risk factors.
- •
The presence of an extrauterine gestational sac containing a yolk sac or embryo (with or without cardiac activity) is the most specific sonographic finding for EP, whereas an echogenic tubal ring in the adnexa is the most common sonographic finding.
- •
When sonography is inconclusive, follow-up with both serum human chorionic gonadotropin (hCG) and transvaginal sonography (TVS) should be used to differentiate an intrauterine pregnancy (IUP) from an EP, as either test alone is often insufficient.
- •
The term pseudogestational sac should be avoided, because it implies the presence of an EP and turns attention away from the fact that in the pregnant patient, most intrauterine fluid collections are IUPs that could be harmed by inappropriate therapy.
- •
The incidence of EPs in unusual locations is increasing. Such EPs are often the result of complications from assisted reproductive techniques (ART) and are associated with higher morbidity and mortality rates compared with tubal EPs.
- •
The term cornual pregnancy has been used variably to describe interstitial EPs (located within the interstitial portion of the fallopian tube as it courses through the myometrium in the cornual portion of the uterus), IUPs located in the angular portion of the endometrial cavity, IUPs in the horn of a bicornuate or septate uterus, or pregnancies in the rudimentary horn of a unicornuate uterus. Hence, the authors recommend that the term cornual pregnancy be abandoned because it is confusing. Rather, a more precise, complete anatomic description that clearly states if the pregnancy is within or separate from the endometrial cavity should be provided for any eccentrically located pregnancy in the cornual region of the uterus.
The most accurate definition of an EP is a pregnancy that has implanted in a location other than within the endometrial cavity ( Fig. 33-1 ). In the United States, approximately 2% of all pregnancies are ectopic in location. Worldwide, EP is a significant cause of morbidity and death in women of child-bearing years, especially in countries or areas with poor prenatal care. The mortality rate from EP has decreased in recent years likely in large part attributable to technologic advances in ultrasound imaging, earlier sonographic imaging and diagnosis, more sensitive hCG tests, and improved treatment with laparoscopy and methotrexate (MTX), along with heightened awareness of the diagnosis among clinicians and patients. However, despite the recent improvements in technology and health care, many cases of EPs are still difficult to diagnose, and some cases elude even the best diagnosticians. Optimal care requires balancing an early but possibly incorrect diagnosis and intervening with the progress of a normal IUP, particularly if it is a desired pregnancy, versus waiting too long to be certain and thus endangering a woman’s life from rupture of an EP. This chapter will review the role of sonography in the diagnosis and management of EP, including EPs in unusual locations.

Epidemiology
According to data from the Centers for Disease Control and Prevention (CDC), the incidence of EP in the United States rose from 0.5% of all pregnancies in the 1970s to 2% in the 1990s. This rise has been linked to an increase in the number of patients with risk factors as well as an increase in the number of pregnancies resulting from ART. However, the reported increased incidence of EP is also almost certainly related to earlier presentation of many patients with EP as well as improved diagnostic techniques, including more sensitive assays for hCG and high-resolution TVS. As a result, some very early EPs are now diagnosed that in years past might have spontaneously resolved without coming to medical attention.
Fortunately, the mortality rate associated with EP has progressively decreased in recent years. The EP mortality ratio in the United States decreased from 1.15 deaths per 100,000 live births in 1980 to 1984 to 0.50 death per 100,000 live births in 2003 to 2007. Based on the current average annual rate of decline, the ratio is expected to further decrease to 0.36 death from EP per 100,000 live births by 2013 to 2017. Nonetheless, recent epidemiologic studies suggest that 6% of maternal deaths in the United States are caused by EPs, and EP remains the leading cause of pregnancy-related death during the first trimester.
Risk Factors
The most common predisposing risk factor for EP is abnormal tubal anatomy, usually tubal scarring, which is most often secondary to salpingitis associated with pelvic inflammatory disease (PID) from sexually transmitted infections ( Table 33-1 ). Salpingitis isthmica nodosa, which is characterized by nodular thickening of the isthmic portion of the fallopian tube accompanied by multiple diverticula, is found in more than 50% of surgically excised tubal EPs. The diverticula correlate well with the location of the EP implantations. Prior history of EP is also a major risk factor. The rate of recurrent EP is 5% to 20% after a single prior EP, but increases to greater than 30% in women with a history of two consecutive EPs. Other causes of tubal scarring include tubal sterilization, reconstructive tubal surgery, and tubal abnormalities related to diethylstilbestrol (DES) exposure in utero. One study reported the risk of EP within 10 years after tubal sterilization to be 7 per 1000 procedures.
| Major Risk Factors |
|
| Minor Risk Factors |
|
In addition, the incidence of EP is increased in women who have conceived using ART, especially following in vitro fertilization (IVF), even if the fallopian tubes are anatomically normal. Tubal pregnancies may result from inadvertent direct placement of embryos into a diseased tube during embryo transfer (ET). Retrograde migration of a transferred embryo from the uterine cavity into an abnormal tube may also occur. IVF patients are at increased risk of EP because of the high prevalence of tubal damage, the use of ovulation induction therapy, and multiple ETs, which put the patient at risk for heterotopic implantations. The presence of an intrauterine device (IUD) increases the risk for EP by a factor of 2.5. An estimated 25% to 50% of pregnancies conceived with an IUD in place are ectopic, and past IUD use may also pose a small increased risk for EP. In general, infertility seems to be a risk factor. There has been no documentation of a clear association between EP and oral contraceptive use, previous elective pregnancy termination, or spontaneous miscarriage.
Other factors with less clear-cut associations have been implicated as risk factors for EP. Epidemiologic studies have shown that cigarette smoking at the time of conception is a risk factor for tubal EP. Possible explanations for this relationship include altered tubal contractility and abnormal ciliary motion from the effect of nicotine or reduced immunity in cigarette smokers, which may affect tubal response to inflammation, as in PID. Endometriosis is also associated with higher rates of EP and miscarriage, possibly because endometriosis and other adjacent inflammatory conditions, such as acute or ruptured appendicitis, can secondarily affect the fallopian tube, thereby causing scarring and adhesions. However, more recent studies claim that there is no association between endometriosis and EP. Abnormalities of the zygote and endocrine dysfunction that may affect ovum transport also increase the risk for EP. Age has also been implicated as a risk factor. Women who have their first sexual encounter when younger than 18 years of age or their first pregnancy when older than 35 years of age have a slightly increased risk of tubal pregnancy. Importantly, however, it should be recognized that approximately 50% of patients with EP have no known risk factors.
Pathophysiology
Approximately 95% of EPs are located within the fallopian tube ( Figs. 33-1 and 33-2 ): 70% in the ampullary portion of the tube, 12% in the isthmic portion, 11% in the fimbriated end, and 2% to 4% in the interstitial portion. Abnormal locations within the uterus include the cervix, a rudimentary horn, and myometrial scars, including scars following cesarean delivery or myomectomy. Other unusual types of EP include intraovarian, abdominal, retroperitoneal, and mediastinal, as well as chronic and heterotopic (in which there is an ectopic as well as an intrauterine gestation) EPs. EP has also rarely been reported after supracervical hysterectomy and even after total abdominal hysterectomy.
Histologically, the fallopian tube is comprised of mucosal, muscularis, and serosal layers. There is no decidua or decidua basalis as in the endometrium. The syncytiotrophoblast and cytotrophoblast of the EP invade into the muscularis layer of the fallopian tube and in essence create adherent trophoblastic tissue. As the trophoblastic tissue grows and the tube stretches, it becomes prone to rupture. Some tubal pregnancies are expelled through the fimbriated end of the tube if the trophoblastic tissue is sheered from its attachment to the muscularis layer, resulting in a tubal abortion. Most of these do not survive, but rarely an abdominal pregnancy may result. If the EP implants outside the tube, then the syncytiotrophoblast and cytotrophoblast will invade underlying tissues and the placental tissue becomes extremely difficult to separate from these tissues, resulting in a substantial increased risk of hemorrhage.
In general, it is helpful to consider two broad categories that give rise to ectopic implantation. The first includes conditions that interfere with transport of the fertilized ovum to the endometrial cavity. The second includes conditions that predispose to premature implantation of the fertilized ovum before reaching the endometrial cavity. The fallopian tube should not be viewed as a passive conduit, but rather is part of a complex interactive process involving tubal peristalsis, ciliary motion, tubal epithelium, and flow of tubal secretions along with the sperm, oocytes, and the conceptus. This all contributes to successful movement of the fertilized ovum through the fallopian tube into the endometrial cavity. This process may become disrupted or altered at many points along the way and result in abnormal implantation. Scarring from prior episodes of salpingitis, tubal sterilization, or tubal reconstructive surgery as well as tubal conditions such as salpingitis isthmica nodosa and abnormalities following in utero DES exposure have been postulated to interfere with transport of the conceptus to the endometrial cavity. Conditions that predispose to premature implantation are less well understood. Previously it was believed that embryos that implant ectopically were likely to have abnormal karyotypes; however, no good data support this theory. A study of 30 surgically excised EPs found no difference in the karyotypes of the chorionic villi from the EPs compared with a control group of IUPs.
Clinical Presentation
Women with EPs most commonly present with symptoms 5 to 8 weeks after the last normal menstrual period. The classic presenting clinical triad of pelvic pain, vaginal bleeding, and an adnexal mass is nonspecific and is found in 20% to 40% of patients with EP. Many patients with pain and vaginal bleeding are not pregnant, and such symptoms are commonly due to hemorrhagic or ruptured ovarian cysts, PID, or dysfunctional uterine bleeding. Even with the addition of a positive pregnancy test and amenorrhea, such signs and symptoms can occur in the setting of a normal IUP, miscarriage, or gestational trophoblastic disease. In fact, it is estimated that 70% to 90% of pregnant women who present with pain or vaginal bleeding in the first trimester and who are clinically suspected of harboring an EP turn out to have an IUP. Clinical findings may also include abdominal or adnexal tenderness. Less common presenting symptoms include referred shoulder pain from irritation of the peritoneal surface under the hemidiaphragm due to free intraperitoneal hemorrhage ( Fig. 33-3 ), cervical motion tenderness, or the urge to defecate secondary to blood collecting in the cul-de-sac. Signs of rupture include hemodynamic instability, orthostatic hypotension, vertigo, loss of consciousness, syncope, or shock from blood loss. EPs in unusual locations may manifest with findings outside the pelvis.

However, tubal EPs can be very difficult to diagnose at an early stage. Nearly a third of cases exhibit no clinical signs and 9% have no symptoms prior to rupture, which can occur at any stage without warning. Frates and associates found that neither sonographic findings nor serum hCG levels were useful predictors of tubal rupture.
Hence, because of the lack of specificity of clinical presentation and physical findings as well as the absence of known risk factors in up to 50% of patients with EPs, clinicians should have a high index of suspicion for EP in any woman of reproductive age who presents with pelvic pain, vaginal bleeding, or symptoms suggestive of blood loss such as syncope or vertigo, even if her pregnancy status is not known. Certainly, any sexually active woman with lower abdominal/pelvic pain and vaginal bleeding after missing a menstrual period should be considered to have an EP until proved otherwise.
Diagnostic Tests
Human Chorionic Gonadotropin
hCG from the blastocyst is first detectable in maternal serum 6 to 8 days following fertilization. Most current urine pregnancy test kits yield positive results 3 to 4 days after implantation. By 7 days after implantation, which is the time of expected menses, 98% of urine pregnancy tests will be positive. Current pregnancy tests are immunoassays of hCG based on monoclonal antibodies to the β-subunit of hCG and are highly sensitive and rapid, with virtually no cross-reactions. If the urine test is negative, suspicion for EP is lowered, but not erased, because a urine pregnancy test will not detect levels of hCG below 25 mIU/mL. Importantly, a quantitative serum hCG test can detect levels approaching zero. If the serum hCG is negative, then an EP is almost certainly excluded, although chronic EP is still possible and rupture of an EP has been reported after the hCG dropped to zero. However, both of these conditions are extremely rare.
There has been much discussion in the literature regarding discriminatory serum hCG levels above which an IUP should be definitively visible by TVS. Levels of 1000 to 2000 mIU/mL (1st International Reference Preparation [IRP]) were previously used as discriminatory levels for expected visualization of an IUP on TVS. However, most researchers and practitioners now agree that no single serum hCG level should be used to differentiate an IUP from an EP. Rather, analysis of serial hCG levels and follow-up TVS are used to reach the correct diagnosis. That said, if the serum hCG level is above 3000 mIU/mL (1st IRP) and the uterus is empty, a normal IUP is unlikely, although there have been anecdotal reports of delivery of a normal pregnancy following an initial TVS demonstrating an empty endometrial cavity with hCG levels as high as 4336 mIU/mL. There are several possible explanations for this shift in discriminatory threshold levels. First, the incidence of multiple gestations has increased, mainly as a result of ART, and hCG levels for a given gestational age would thus be higher in a woman carrying a multiple gestation, compared to a singleton pregnancy, upon which earlier discriminatory numbers were derived. Second, data upon which earlier discriminatory numbers were based came from single institution studies with small patient populations. Third, clinical management and treatment have changed. Now, MTX is often given to treat EPs. If an erroneous, false positive diagnosis of EP is made and an IUP is not recognized, treatment with MTX can result in serious harm to the developing embryo. Hence, the current goal is to achieve 100% specificity with no false positive diagnoses of EP.
In general, the serum hCG level is lower for a given gestational age in the setting of an EP than with an IUP. Hence, when clinical findings are suspicious for possible EP, TVS is indicated even if the serum hCG is very low. Approximately 70% of women with EPs have a slow rise in serum hCG, slower than expected for a normal IUP, or a slow fall in hCG, slower than typical for spontaneous miscarriage. Hence, a suboptimal increase (i.e., <53% in 48 hours) or plateau of serial hCG values suggests an EP. Of note, serum hCG levels in some women with EPs may be similar to expected hCG levels in women with normal IUPs. One study revealed that up to 27% of women diagnosed with EP had hCG curves resembling normal IUPs. Others have found that 15% to 20% of EPs have hCG doubling times similar to those for IUPs. In addition, studies have shown that serum hCG levels may increase by only 35% to 66% over 48 hours for some normal IUPs, rather than doubling in that time frame, as has been previously expected. Furthermore, in 8% of spontaneously resolving EPs, the serum hCG level will fall in a pattern mimicking spontaneous miscarriage. As a result, the use of serum hCG levels alone for follow-up is unreliable for the diagnosis and management of EP.
Using sonography alone for follow-up is also not reliable because some EPs are too small to visualize. Additionally, 6% of apparent miscarriages on ultrasound examination may actually have an underlying EP. A presumed completed miscarriage based on TVS findings of an empty uterus should be treated as a pregnancy of unknown location (PUL) until confirmed as a miscarriage based on serial hCG levels and follow-up TVS. There are reports of patients discharged to home with presumed miscarriage subsequently returning with pain, bleeding, and ruptured EP.
PUL is a descriptive term referring to a pregnancy that is detected biochemically (positive pregnancy test), but not yet visible sonographically or laparoscopically. This term, a temporary descriptor, is used as a holding pattern until the location of the pregnancy is established. The incidence of PULs is dependent not only upon the expertise of the examiners and the adequacy of the sonographic evaluation but also upon gestational age. As women present earlier in pregnancy, more PULs are reported. In 1999 Banerjee and colleagues reported an 8% incidence (135 cases) of PULs in their series of first trimester sonograms. On follow-up, 14% of these turned out to be EPs; 27% developed into normal IUPs; 9% resulted in miscarriages; and 50% resolved spontaneously. Kirk and Bourne reported that in 8% to 31% of early pregnancies, the gestation will not be visualized on the initial TVS. The vast majority of PULs are eventually found to be IUPs or failed IUPs, and only approximately 7% to 20% are EPs. However, follow-up is very important. There is no reliable method by which to predict which PULs are IUPs and will either progress normally or miscarry and which PULs are EPs and, of these, which EPs will spontaneously resolve and which are likely to rupture. Unfortunately, rupture of EPs has been reported with declining or very low hCG levels, including some rare cases with negative hCG values. It is therefore incorrect to assume that because the hCG level is low, an EP is unlikely or stable or to assume that an EP is too small to visualize and thus not perform a sonogram (see Fig. 33-3 ).
Molecular Biomarkers
Studies are ongoing in attempts to identify diagnostic molecular biomarkers that can reliably distinguish EPs from both normal and abnormal IUPs at an early stage, when the location of the pregnancy is not yet known. These data would be extremely useful in the early diagnosis of EP, obviating the need for multiple follow-up blood tests, ultrasound examinations, and clinic visits, while at the same time decreasing the risk of EP rupture. One of the main challenges in these investigations is that there is no suitable animal model for tubal EP, so research must be performed directly in women. Biomarkers of trophoblastic function, corpus luteal function, angiogenesis, endometrial function, inflammation, muscle damage, and impaired tubal transport are currently being investigated. Although a variety of serum, plasma, and urine biomarkers have been evaluated both independently and in combination, most do not have adequate discriminatory capability to be clinically useful. One controlled study of patients with EPs and viable IUPs with hCG levels lower than 1500 mIU/mL demonstrated that a four-marker test, including progesterone, vascular endothelial growth factor (VEGF), inhibin A, and activin A, could predict EP with 100% accuracy. However, additional investigations that include patients with abnormal IUPs are necessary to further assess the utility and reliability of this panel of markers.
Transvaginal Sonography
In a patient suspected of having an EP, quantitative serum hCG levels and TVS are used in conjunction for diagnosis. It is reasonable to proceed with TVS while the quantitative hCG is being measured or independent of hCG level. Justification for performing TVS prior to obtaining the serum hCG results includes reports that up to 50% of ruptured EPs have extremely low hCG levels (<1000 mIU/mL) and that hCG levels do not necessarily correlate with risk of rupture.
Although TVS is much more sensitive than transabdominal sonography, most experts recommend that scanning begin with transabdominal pelvic ultrasound, albeit without intentional filling of the bladder. The aim of this limited transabdominal pelvic scan is to obtain an overview of the pelvis; establish the size, location, and position of the uterus; locate an ectopic sac in a high location ( Fig. 33-4 ); and assess for free intraperitoneal fluid, including in the Morison pouch, between the liver and right kidney. This is an excellent method for identifying a large hemoperitoneum that may be missed by TVS. In a supine patient, blood may accumulate in the upper quadrants with only a minimal residual amount of fluid seen in the pelvis. Subsequently, a detailed TVS is performed to provide higher resolution evaluation of the uterus and adnexa.

The yield and reliability of TVS depend upon transducer frequency, patient body habitus, uterine position, presence of leiomyomas, and operator expertise. Optimal transducer frequency for TVS is usually between 5 and 10 MHz. Depth is set such that there is visualization of pelvic structures 2 to 3 cm beyond the margin of the uterus or ovary, so as not to miss an adjacent or exophytic mass. Overall gain and time gain compensation (TGC) curves are set to optimize the image within the field of view. The object of interest is placed within the focal zone, and the use of multiple focal zones improves resolution. Harmonic imaging and spatial compounding will also improve resolution. Magnification of the area of interest within the uterus or adnexa may improve visualization. Gentle bimanual palpation exerted with one hand on the transvaginal transducer and the other hand pressing on the anterior abdominal wall can be helpful and may serve to identify the area of the patient’s pain, where disease is most likely to be found. This maneuver may move structures of interest toward the focal zone such that they are easier to see and will compress bowel, thereby eliminating artifact from air within the bowel lumen. In addition, exerting compression on an adnexal mass and ovary can help differentiate an exophytic corpus luteum, attached to the ovary, from an adjacent EP, which typically move apart, separate from the ovary. Three-dimensional (3D) sonography may be helpful, particularly in evaluation of EPs in unusual locations.
The first structure imaged by ultrasound is the cervix; the endocervical canal should be perpendicular to the ultrasound beam and the walls of the cervix in continuity with the myometrium of the uterine corpus. This positioning allows for evaluation of a possible cervical EP or miscarriage in progress and provides optimal visualization of the cul-de-sac, posterior to the cervix. The cul-de-sac is the most dependent portion of the female pelvis and thus is a common location for EPs or hemoperitoneum. Subsequently, the endometrium, myometrium, and adnexa are thoroughly evaluated. Any fluid within the endometrial cavity is assessed for location, shape, rim, and presence of internal echoes to determine the possibility of an IUP. If an ectopic gestational sac is not identified between the uterus and the ovary, one must scan as far lateral as possible in the pelvis, recognizing that this may result in some discomfort for the patient. Potential “blind spots,” where EPs can be located and are difficult to detect include the pelvic side walls, deep cul-de-sac, above the uterine fundus, and anteriorly between the uterus and bladder. The examination must include targeted evaluation in the region of the patient’s pain. Finally, if the hepatorenal space was not checked at the beginning of the examination, one should keep the patient supine and scan, looking for fluid in the Morison pouch.
Color, power, or spectral Doppler sonography may be used to demonstrate blood flow to an EP and to differentiate between vascularized trophoblastic tissue and avascular hematoma. However, Doppler sonography of the endometrium is not advised in order to avoid insonating a possible early IUP that is not yet visible. Color Doppler ultrasound parameters should be optimized for the detection of slow flow. This includes decreasing the scale or pulse repetition frequency (PRF), increasing gain and color saturation, decreasing the wall filter, setting higher color priority, and using a small color box. Spectral Doppler tracings should be obtained to confirm blood flow demonstrated on color Doppler images, as color Doppler sonography is sensitive to motion, which may create color artifact mimicking blood flow.
Sonographic Diagnosis of Ectopic Pregnancy
Uterine Findings
In the setting of EP, sonographic findings in the uterus are nonspecific and include absence of an intrauterine gestational sac and fluid in the endometrial canal. If there is no visible intrauterine gestational sac, diagnostic possibilities include EP, very early IUP, failed IUP, or heterotopic pregnancies. At this point, the pregnancy is deemed to be a PUL, and, in current practice, the diagnosis of EP should be based upon positive findings rather than on the absence of an intrauterine gestational sac with serum hCG above a specific discriminatory level. Hence, the search continues. Although the presence of an IUP makes the probability of an EP much less likely, heterotopic pregnancies have been reported in 1 out of 4000 pregnancies in the general population, and the incidence is as high as 1 in 100 to 300 pregnancies in women who have undergone ART.
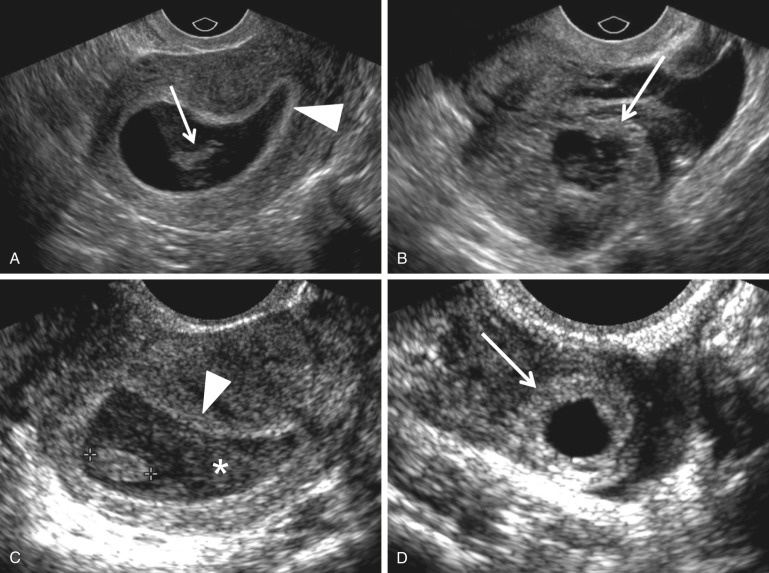
Endometrial thickness has not been shown to reliably predict the presence of an EP or the outcome of a PUL. Likewise, decidual cysts have not been proved to be predictive of EPs, despite initial reports that a thin-walled endometrial cyst was associated with a high risk for EP ( Fig. 33-5 ). Although Laing and coworkers reported that a thin-walled decidual cyst can be differentiated from an early intrauterine gestational sac with its thicker echogenic wall (intradecidual sac sign) ( Fig. 33-5B ), most authors rely on the identification of a yolk sac within an endometrial fluid collection to definitively diagnose an intrauterine gestational sac.

In the past, it was thought that intracavitary blood or secretions were relatively common findings in patients with EPs. This fluid, often containing low level echoes, located centrally within the uterine cavity and surrounded by a single echogenic decidual layer, was termed a “pseudogestational sac” or “pseudosac” ( Fig. 33-6 ). Thus, it could be differentiated from a true intrauterine gestational sac, which is either eccentric and embedded in a single layer of the endometrium (intradecidual sign) or surrounded at least partially by two echogenic rims of tissue with an interleaving hypoechoic layer (the double decidual sac sign). However, at present, fluid in the endometrial canal is observed in only 10% to 20% of women with EPs, possibly because of sonographic evaluation at earlier gestational ages. Benson and associates report that in current practice any round or oval well-defined fluid collection in the mid to upper endometrial cavity is statistically more likely to represent an IUP (99.98%) rather than a “pseudosac” associated with an EP (0.02%). Note that an endometrial fluid collection with a tapered or pointed edge is much more likely to be associated with an EP (see Fig. 33-6 ). In conclusion, the terms “pseudosac” and “pseudogestational sac” should probably be abandoned as they are confusing and of limited use. In current practice, endometrial fluid is infrequently observed in patients with EP.

Adnexal Findings
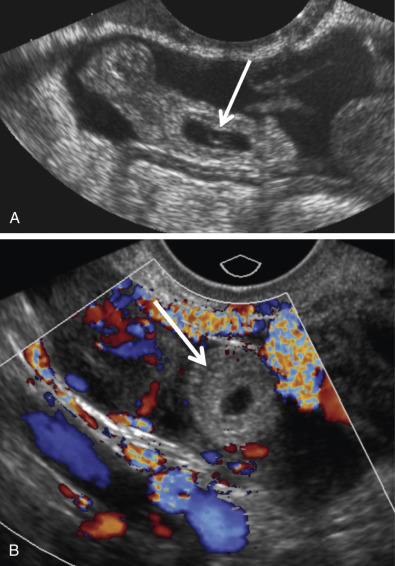
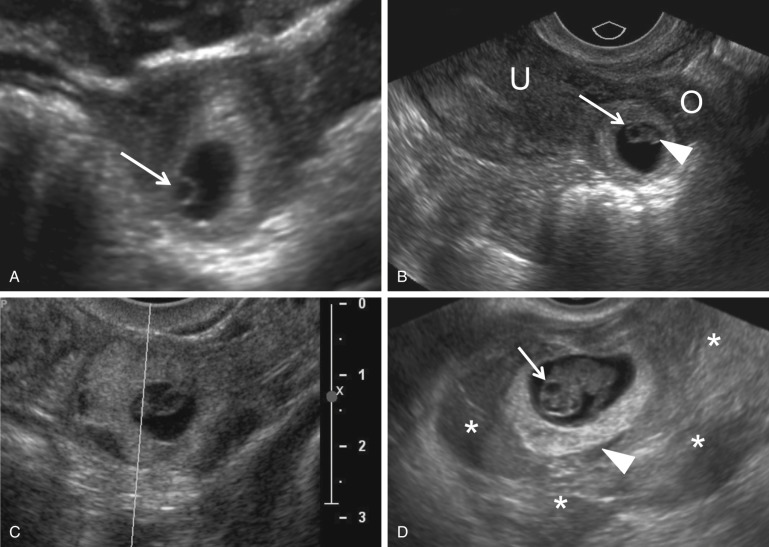
Visualization of an extrauterine gestational sac containing a yolk sac or an embryo with or without a heartbeat is diagnostic of an EP with 100% positive predictive value (PPV) ( Fig. 33-7 ). The next most reliable sonographic finding is an adnexal tubal ring representing an empty gestational sac that appears as a round anechoic fluid collection with a thick echogenic rim. This has also been described as the bagel, donut, or sugar donut sign ( Figs. 33-2B, 33-3A, and 33-8 ). The adnexal tubal ring sign has a 95% PPV for EP and is seen in approximately 50% of cases. The PPV increases if the tubal ring is clearly separate from the ipsilateral ovary ( Fig. 33-8B ). Color Doppler sonographic imaging may increase the conspicuity of a small tubal ring ( Fig. 33-8C ). Although some EPs will demonstrate circumferential vascularity, most EPs will demonstrate only segmental or focal vascularity, and some may demonstrate no flow at all on color Doppler ultrasound imaging ( Figs. 33-2B and 33-6C ). EPs may also appear as a nonspecific adnexal mass representing hemorrhage with blood clot. These masses can be homogeneous, heterogeneous, or complex; may appear either predominantly cystic or solid; and have variable echogenicity ranging from hypo- to hyperechoic, depending upon the time course since hemorrhage occurred ( Fig. 33-9 ). Hemorrhage contained within the fallopian tube (hematosalpinx) will appear tubular or ovoid in shape ( Fig. 33-10 ). Internal vascularity increases the likelihood that the mass represents an EP rather than bland hemorrhage from a ruptured corpus luteum. In the setting of a positive pregnancy test and an empty uterus, any adnexal mass that is clearly not either an intraovarian corpus luteum, paraovarian cyst, or pedunculated myoma has a 92% PPV for EP. EPs are most commonly found situated between the ovary and uterus or in the cul-de-sac, which is the most dependent portion of the female pelvis. Although rare, it is possible to occasionally see twinning in ectopic locations. Possibilities include twin sacs in one fallopian tube, one twin in each tube, monochorionic twins, and conjoined twins ( Fig. 33-11 ).




Cul-de-Sac Findings
Echogenic free fluid indicative of hemoperitoneum may be the only initial finding in up to 25% of patients with EPs ( Fig. 33-12 ). Hemoperitoneum typically collects in the cul-de-sac as this is the most dependent portion of the female pelvis in the upright position. A pregnant woman with an empty uterus has a 90% probability of EP if a moderate to large amount of echogenic free peritoneal fluid is found. However, the volume of free fluid has low sensitivity, specificity, and PPV for determining tubal rupture. The amount of free fluid is a good indicator of the risk of hemodynamic instability. Therefore, the upper abdomen should always be assessed as the presence of free fluid in the Morison pouch suggests that there is a relatively large volume and would increase concern for potential hemodynamic instability. When evaluating free fluid, careful attention should be paid to technique as high gain settings can create artifactual echoes. Comparison of cul-de-sac fluid with known anechoic fluid such as urine in the bladder or fluid in an ovarian cyst/follicle at similar depth will help reveal whether echoes are real or artifactual ( Fig. 33-12B ). One can also look for layering or movement of low-level echoes. It should be remembered that on occasion acute hemoperitoneum may appear as anechoic fluid. Occasionally, hemoperitoneum in the Morison pouch may appear so echogenic that it approximates the echogenicity of adjacent hepatic parenchyma and may be mistaken for liver because of the silhouette effect ( Fig. 33-13 ). Also, clotted blood may appear echogenic and mass-like, and thus may be difficult to differentiate from loops of bowel ( Fig. 33-12D ). After the initial clotting stage, fibrinolysis begins and hemoperitoneum will appear heterogeneous, making it more difficult to visualize a small ectopic gestational sac amid amorphous material in the cul-de-sac (see Figs. 33-3 and 33-12B ).


Pitfalls
General limitations in the sonographic diagnosis of EP include lack of operator experience, training, skill, persistence, or lack of attention to details. Patient obesity, leiomyomas, uterine anomalies, retroflexion of the uterus, or hydrosalpinx may also limit sonographic evaluation. Careful and complete patient history should address risk factors such as prior EP, ART, PID, or tubal surgery. If the patient presents at an early gestational age, the EP may be too small to visualize and may fall below the limits of resolution of the ultrasound equipment.
Potential pitfalls in image interpretation include misconstruing fragments of decidua or blood clot within an intrauterine fluid collection as either a yolk sac or embryo, thus leading to a false positive diagnosis of IUP ( Fig. 33-14 ). It can also be very difficult to differentiate an exophytic corpus luteum from an EP ( Fig. 33-15 ), particularly because 80% of EPs will be located ipsilateral to the ovarian corpus luteum ( Fig. 33-16 ). There are several useful points of distinction ( Fig. 33-17 ). A corpus luteum arises from the ovary, and intraovarian EPs are extremely rare (see later). Although a corpus luteum can be exophytic, a peripheral crescent of ovarian parenchyma, termed the “claw sign,” will typically be observed surrounding at least part of the corpus luteum. However, if a mass adjacent to the ovary represents an EP, it will be separate from the ovary with an acute intervening angle between the structures and no “claw sign” (see Fig. 33-17 ). When prodded with the transvaginal probe or with palpation on the anterior abdominal wall, the EP will typically slide away from the ovary, whereas a corpus luteum will remain attached to and move with the ovary. The tubal ring of an EP is typically thinner and more echogenic than the homogeneous thick wall of the corpus luteum ( Figs. 33-15A, 33-15C, 33-16A, and 33-17C ). However, there is considerable overlap in the echogenicity and thickness of the walls of a corpus luteum and an EP. As a corpus luteum involutes, the inner margin may become crenated or stellate in appearance ( Fig. 33-15A and B ). Internal echoes are relatively common as a result of hemorrhage and may mimic a yolk sac or embryo (see Fig. 33-15B ). Magnified views using harmonic imaging or spatial compounding may be helpful given that definitive identification of a yolk sac or embryo in an adnexal ring-like structure confirms the diagnosis of EP. Both a corpus luteum and an EP can demonstrate marked peripheral vascularity on color Doppler imaging, termed the “ring of fire,” with low-resistance spectral Doppler waveforms with increased diastolic flow. Neither the degree of mural vascularity depicted on color Doppler ultrasound nor the characteristics of flow on spectral Doppler waveforms is helpful in differentiating an EP from a corpus luteum. Although the “ring of fire” sign was initially described as a pattern of color Doppler flow suggestive of EP, subsequent experience has shown that a complete circumferential (360-degree) “ring of fire” is actually more commonly seen with corpora lutea ( Figs. 33-15B, 33-15D , and 33-16B ). Blood flow in the tubal ring of an EP is more likely to be focal and segmental ( Figs. 33-8C and 33-11A ).