Hot flashes
Vaginal dryness
Night sweats
Overactive bladder
Mood swings
Skin changes
Headache
Hair loss
Insomnia
Irritability
Lack of concentration
Weight gain
Poor memory
Lack of libido
Joint pains
Depression
Apart from the climacteric symptoms, increased cardiovascular disease, cerebrovascular and musculoskeletal deterioration are taken into consideration. Women with an early menopause have a higher incidence of ischemic heart disease and overall mortality risk is reduced with HRT in observational studies [53–55]. Unfortunately, there is little information that allows the clinician to determine the least disadvantageous HRT combination. There is an ongoing research to establish the impact of body identical hormones (particularly the progesterone component) compared to synthetic hormones, suggesting a reduced metabolic impact of estradiol, and natural progesterone compared to synthetic progestogen, in addition to possible reduced breast cancer risk [56]. It seems logical to replace women’s hormones with identical compositions, if possible. Younger women with cervical cancer may wish to have replacement in the form of the combined oral contraceptive pill, although this may have a more disadvantageous metabolic impact. Those who have been hysterectomised do not require progestogen or progesterone to protect the endometrium from oestrogen stimulation, thus conferring no additional breast cancer risk. There is also no additional risk with combined HRT used before the average age of menopause, (approximately age 51). There are practitioners withdrawing progesterone opposition in systemic HRT regimens for women who have had pelvic radiotherapy with an intact uterus; but there is no published evidence to support the safety of this regimen, even though the endometrium is likely to have been ablated in most.
Table 11.2 details HRT that can be administered to those without hormone-sensitive cancers, or who are judged to have minimal risk of recurrence and a significant beneficial impact of oestrogen deficiency on their quality of life. Different routes of administration will have varying metabolic pathways with different risks and benefits. Transdermal oestrogen products in the form of patch or gel have less impact on coagulation, and therefore lower the risk of thrombosis (although the overall risk is low in younger women being treated for iatrogenic menopause compared to older naturally menopausal women). However, pelvic cancer itself confers an increased risk of venous thrombosis. Progestogen can be administered orally or transdermally in a combined patch or using an intrauterine device. Progesterone is available on oral, vaginal or rectal preparations. Androgenic properties of some progestogens may counteract the beneficial effect of estradiol on lipoproteins and increase insulin resistance [57, 58].
Table 11.2
Hormone replacement therapy
Systemic hormone replacement therapy | Topical genital treatments |
|---|---|
Oral combined HRT-—sequential or continuous combined | Oestrogen (oestradiol/oestriol) cream |
Patch combined HRT—sequential or continuous combined | Oestrogen pessary/ovule |
Oestrogen only tablet | Oestrogen ring |
Oestrogen only patch | Replens |
Oestrogen only gel | Hyalofemme |
Oestrogen and testosterone implants | possible benefit from topical DHEA/testosterone |
Testosterone patch/gel | Lubricants |
Tibolone | – |
Progestogen tablet/IUS | – |
Progesterone tablet, vaginal gel/rectal suppository | – |
Although not specifically studied in a treatment group with gynaecological cancer, continuous combined or non-bleeding HRT is safer for the endometrium long-term (i.e. daily dosing of progesterone or progestogen). There may be difficulties in assessing postmenopausal bleeding in this group of women after radiation therapy due to other causes including vaginal and/or cervical stenosis, and changes in the appearance of the endometrium on imaging. Sampling the endometrium may also be difficult in this group.
Topical estrogens are an alternative for promoting vaginal health, with a significant reduction in recurrent cystitis or urinary tract infection that can be provoked by intercourse or dilator use in addition to improvement in vaginal symptoms of itching, burning and irritation, secondary to vaginal atrophy. These agents are safe even in those with hormone sensitive cancers in the vast majority of patients. Vaginal tablets, pessaries, creams and a ring are available for use. These could potentially be used indefinitely for those who are symptomatic and not using systemic HRT. They may also reduce bleeding with dilator use and intercourse. Topical estrogens are minimally absorbed during the first 2 weeks of daily use only. After this, levels are generally consistently below the normal postmenopausal levels.
Consideration of androgen deficiency should also be undertaken, particularly in younger surgically menopausal women. There is data to suggest benefits for women’s sexual satisfaction, well-being, energy levels and musculoskeletal strength, although this is a controversial area in hormone replacement [59, 60]. Tibolone is a gonadomimetic alternative to combined hormone replacement with estrogenic, progestogenic and androgenic effects, and proven benefits for tissues, vasomotor symptoms and sexual functioning [61, 62].
The current recommendations regarding hormone replacement in women with induced menopause after cancer, is to administer HRT until at least the average age of natural menopause, i.e. 51 years. This approach should be assessed on a regular basis in line with current evidence. Alternatives can be considered but oestrogen with or without progestgen/progesterone is the only treatment for all consequences of oestrogen deficiency. Many herbal therapies, such as phytoestrogens, work via pathways that are not clarified, and may have hormone-like effects and therefore exhibit similar risks. Venlafaxine and gabapentin may reduce vasomotor symptoms but cause significant side effects for some [63]. Bisphosphonates can be used for prevention and treatment of osteoporosis, but long-term use is associated with fragility fractures. All these women should be counselled with respect to maintaining their cardiovascular and bone health by weight-bearing exercise, sufficient calcium and vitamin D and good diet with screening for bone density and cardiovascular risk factors as required.
Fertility
In the last 20 years, an increasing number of women have been long-term survivors after radiotherapy for cancer [64]. In addition, radiotherapy is used successfully for other conditions such as autoimmune or hematological and CNS conditions. This, in combination with the fact that women tend to postpone childbearing for social reasons, means that many want to start a family well after receiving radiotherapy. Many want to be informed about the effects of radiotherapy on their options for fertility and age of menopause, as well as future pregnancies and neonatal outcome.
Ovarian Reserve
The term ovarian reserve describes the quality and quantity of ova in a woman’s ovaries at a specific age. Women are born with a finite number of oocytes, usually around 1–2 million, and by the age of menarche they are left with 500,000. The follicles are lost exponentially, mostly with atresia, and reach a number of around 25,000 at an average age of 37–38, and less than 1000 at menopause, normally around the age of 51. However, there is huge variability in these numbers at any age. The rate of follicle loss depends mainly on the number of remaining follicles, and accelerates towards the end of reproductive life. There is no direct way to assess the ovarian reserve. Most so-called tests of ovarian reserve tests basically predict ovarian response to stimulation with gonadotrophins and, at best, they provide quantitative estimates. Antral follicle counts and animullerian hormone measurements are the most accurate. By extrapolation, they can provide a rough prediction of the age of menopause, but they cannot assess a woman’s current level of fertility as this is dependent mainly on the quality, not the number of eggs.
Early Ovarian Ageing
Early ovarian aging is a term introduced in 2003 [65] to describe women who are destined to develop menopause before the age of 46, having started an accelerated decline of their ovarian reserve from their early 30s. Most of these women were actually born with significantly fewer oocytes in their ovaries than the average woman, and the reasons for this are usually genetic. However, there are a number of acquired causes of early ovarian aging, which act through reductions of the ovarian reserve. These include radiotherapy, chemotherapy and surgery. Detection of asymptomatic women with early ovarian aging in their late 20s or early 30s may be possible by screening of high-risk groups.
Effects of Radiotherapy on Ovarian Function
Radiation causes direct damage to the ovarian reserve by destroying follicles. The most vulnerable of these are the growing follicles, whereas primordial follicles seem to be relatively protected. The extent of damage to the ovarian reserve depends on the patient’s age, the dose of radiation and the field of radiation. Mathematical models have been developed to assist physicians to predict the likely effect of radiation to the ovaries on the ovarian reserve, in order to counsel the patients and their families [66]. It has been estimated that the radiation dose that will destroy half of the existing immature oocytes is 2Gy (LD50). Injury is worse with higher doses of radiation. In addition, older patients or patients with poor ovarian reserve are more sensitive to these radiation effects. The extent of the radiation field has an effect on the degree of ovarian damage. With total-body irradiation, 90 % of patients have been observed develop premature ovarian failure. This increases to 97 % in patients receiving abdominal radiation.
Effects of Radiation on the Hypothalamic-Pituitary-Ovarian Axis
Many studies have been performed to determine the effect of cranial or whole-body irradiation on the pituitary-hypothalamic-ovarian axis [67, 68]. Damage seems to occur at the level of the hypothalamus and pituitary gland. Subsequent hormonal alterations include hypogonadism and oligomenorrhoea, but also low lutenizing hormone and prolactin levels. There seems to be a long-latent period for these abnormalities; therefore, women who have received radiotherapy should be monitored for these potential abnormalities for several years after radiation exposure.
Effects on the Uterus
Pelvic irradiation puts women at increased risk of pregnancy-related complications, such as miscarriage, intrauterine growth retardation, preterm labour; and placental abnormalities such as placenta accreta [69]. The uterus of women post irradiation decreases to 40 % of the normal size, and also the endometrium becomes relatively thin. Radiation causes damage to the uterine muscle with fibrosis and, this affects uterine expansion during pregnancy. It also produces potentially chronic damage of the uterine blood vessels. The extent of uterine damage depends on the dose of radiation, the radiation field and the age of the patient. The pre-pubertal uterus is much more sensitive to radiation effects. It is estimated that the dose required to cause uterine dysfunction is around 14Gy.
Strategies to Minimize Radiation Damage
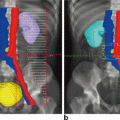
Ovarian transposition or oophoropexy is an operation to move the ovaries away from the uterus and pelvis in order to spare them from radiation exposure. It was introduced in the 1950s for cervical cancer patients, but is now considered in many other cases, including Hodgkin’s lymphoma, pediatric sarcomas and rectal cancer. Traditionally, it was performed during laparotomy for radical hysterectomy or staging for Hodgkin’s lymphoma, but is now also performed laparoscopically. The ovary and fallopian tube are dissected from the uterus and attached to the peritoneum laterally and as high as possible. Studies have shown this technique to be effective [69]. Ovarian shielding is also used, depending on the required field of radiation. Newer radiotherapy protocols are generally more effective and cause smaller collateral damage. Another approach is pre-treatment with GnRH analogues, which seem to offer some protection for the ovarian reserve and endometrium.
Fertility Preservation
There are various possibilities for fertility preservation for pre-menopausal women who have a risk of ovarian injury [70]. The most effective of these is embryo freezing, but this requires in vitro fertilization (IVF) with partner sperm or donor sperm. IVF is not always feasible and may be contraindicated. Egg-freezing does not require sperm rather is a much newer technique, and the possibility of a live birth is far less certain. Ovarian tissue cryopreservation is another possibility, which can also be used for pre-pubertal women. This is becoming more successful, although the number of known live births is still very small. Women can try to conceive using donated eggs or a surrogate mother’s uterus, but obviously this involves ethical and legal issues that need to be discussed [70, 71].
Best Practice
As the survival of female cancer patients continues to improve, it is critical to consider the long-term reproductive health and fertility of patients when planning radiotherapy and other treatments for their cancers. This is achieved through a multidisciplinary approach, involving medical and radiological oncologists, gynaecologists and paediatricians. It is important to individualize treatments including methods to minimize damage to the ovarian reserve and uterus. The issue of future fertility should be discussed with the patient prior to radiotherapy. Possible options for the minimization of damage to the female organs and fertility preservation should be reviewed. Women and their families should be offered counselling and other services to assist with preservation of sexual, health and overall well being.
Conclusion
There are significant acute and chronic effects of radiation treatment for pelvic cancer. It is imperative that healthcare professionals inform their patients of these effects, including lifestyle-related and sexual side effects that may occur. Recognition of patient fears and concerns, and the consequent adaptive behaviours are needed by practitioners. Information, given in both verbal and written forms will provide support for these women. An understanding of hormonal changes and minimization of their consequences are required to preserve quality of life. Research with validated instruments needs to be undertaken to enhance the understanding of sexual dysfunction and fertility issues in these patients. Better information will improve patient decision-making. Quality of life issues must be addressed in patients undergoing treatment for gynaecological malignancies as increased survivorship necessitates more research to facilitate overall improved health care for these women.
References
1.
2.
Mercer CH, Fenton KA, Johnson AM, Wellings K, Macdowall W, McManus S, Nanchahal K, Erens B. Sexual function problems and help seeking behaviour in Britain: national probability sample survey. BMJ. 2003;327(7412):426–7.CrossRefPubMedCentralPubMed
3.
White ID. The assessment and management of sexual difficulties after treatment of cervical and endometrial malignancies. Clin Oncol (R Coll Radiol). 2008;20(6):488–96.CrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree