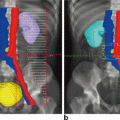
Fig. 10.1
Mechanisms involved in the complex physiopathology of anal incontinence secondary to pelvic irradiation
Radiation Proctopathy
Acute radiation proctopathy develops during or shortly after a course of radiation therapy. It presents as diarrhea, rectal pain, and tenesmus and is usually of short duration.
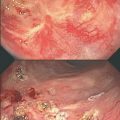
Chronic radiation proctopathy occurs at least 6 months after radiotherapy; about 85 % of cases present within the first 2 years after radiotherapy. Although the true incidence of chronic radiation proctopathy is unknown, data from retrospective studies suggest that between 2 and 20 % of patients who receive radical pelvic irradiation are at risk of developing chronic radiation proctitis [5]. This condition is characterized by painless passage of blood per rectum, mucous rectal discharge, frequent bowel movements, urgency, and fecal incontinence rectal pain. Less commonly, severe rectal bleeding, ulceration, fistulas, bowel perforation, and bowel obstruction can occur.
Late radiation damage is often characterized histologically by a loss of parenchymal cells and an overproduction of collagen. The classic theory of late radiation injury states that depletion of parenchymal cells leads to late injury, and that the latent period preceding the development of late effects is caused by the long cell cycle of many of these target cells. Haboubi et al. [6] reported patients who underwent rectal biopsies during or shortly after completing radiotherapy (6 patients) or 4 months after radiotherapy (4 patients). Biopsies from the former group showed epithelioid meganucleosis, lack of mitotic activity, and patchy fibroblastic activity in the lamina propria, whereas the blood vessels appeared normal. Biopsies from the latter group showed subintimal fibrosis resulting in arteriole narrowing, telangiectasia of capillaries and postcapillary venules, endothelial degeneration, platelet thrombi formation, fibrosis of the lamina propria, and crypt distortion. These authors suggested that cellular epithelial change and fibroblastic proliferation occur initially followed by vascular changes.
Recent evidence has highlighted the importance of microvascular endothelial damage as a major contributor to normal tissue injury after radiation [7, 8]. The endothelium has been shown to be an important target for radiation in the lung, brain, and gut; apoptosis of the microvascular endothelial cell seems to be the earliest lesion in the gastrointestinal tract after radiation followed by stem cell dysfunction [7].
Effects of Pelvic Radiation on Anorectal Function
The most commonly irradiated pelvic organs are the prostate and cervix, followed by the anus [2]. The adverse effects of pelvic radiation in the anorectal sphincter (both muscular and neurogenic) and rectal reservoir capacity and compliance have long since been documented [1, 2]. Rectal cancer is a relatively common malignancy and more recently the role for neoadjuvant chemoradiation has been defined. Currently, patients undergoing treatment for rectal cancer require a multimodality approach. The adverse effects on anorectal function are very complex due to the combined effects of chemoradiation as well as the rectal resection.
Effects of Surgery
In the last decade, sphincter-preserving techniques have replaced abdominoperineal resection and a permanent stoma in patients with midrectal and low rectal cancer, as long as the mesorectum is totally excised and an adequate distal margin of 2.0 cm can be obtained. Therefore, the low and ultra-low coloanal anastomosis can be accomplished, and procedures such as intersphincteric resection of the rectum have been developed. However, when the level of the anastomosis becomes less than 3–4 cm to the dentate line, the patient’s quality of life may be significantly compromised. In these patients, in addition to the loss of the rectal reservoir, the resting pressure barrier can be greatly compromised by damage to or removal of the internal anal sphincter. Therefore, the occurrence of incontinence in these patients is influenced by diminished rectal capacity and compliance, decreased internal anal sphincter tone, and impairment of rectoanal inhibitory reflex and anal sensitivity.
Neural injury after rectal resection, is manifested by a variety of symptoms, including urinary dysfunction (as high as 12 % of patients) and sexual dysfunction, in 10–35 % of patients after total mesorectal excision. New techniques have been designed to minimize these complications [9–11], particularly the introduction of the nerve sparing total mesorectal excision technique. The goal of these techniques is to provide negative circumferential margins while minimizing the incidence of complications due to neural injury, such as genitourinary dysfunction. Although only limited data are available regarding robotic-assisted total mesorectal excision, this recent innovative technique may improve functional outcome due to better preservation of autonomic nerves [12]. However, due to its cost, further studies are warranted to justify its role in routine application.
Restorative protectomy can adversely affect the mechanisms of anal continence in 30–90 % of patients after anterior resection. This condition is known as the anterior resection syndrome [13]. Symptoms include increased bowel frequency, urgency, fragmentation of stool and fecal incontinence, and have a negative effect on patient’s quality of life. Multiple factors may produce the anterior resection syndrome. Although the loss of rectal reservoir and its mechanisms of capacity and compliance have been largely implicated, sphincter damage and autonomic nerve injury from surgery are other possible causes [14, 15]. Whenever the rectum is removed and a low colorectal or coloanal anastomosis is necessary, the option of adding a colonic pouch or a coloplasty should be considered. Although the functional results of this “neorectal reservoir” are controversial, a recent meta-analysis demonstrates equally beneficial effects on anorectal functional symptoms [16].
Radiotherapy adversely affects rectal function due to reduction in rectal capacity and compliance as a consequence of proctitis and fibrosis of the rectal wall [3, 17]. Preoperative or neoadjuvant chemoradiotherapy can minimize this problem because the irradiated rectum is excised and healthy colon is anastomosed to the lower rectum or anus. Intraoperative radiotherapy has also been employed to diminish impairment of anorectal function. Postoperative incontinence appears to be most pronounced when postoperative external beam radiotherapy was administered [18, 19].
Varma et al. [20, 21] studied anorectal manometeric findings in patients with prostate cancer receiving external beam radiation. They also reviewed histopathological aspects of formalin-treated tissue from the lower rectum obtained from eight other patients who had undergone excisional surgery for complications of radiation rectal injury. In these patients, damage to the myenteric (Auerbach’s) plexus was demonstrated, and included marked hypertrophy of the nerve fiber with vacuolation of the nerve sheaths and changes in number (decreased) and morphology of ganglion cells. Although the function of the external anal sphincter remains relatively unaffected, dysfunction of the internal anal sphincters seems to be the main factor in the pathophysiology of anorectal dysfunction. Damage to the radiosensitive myenteric plexus is an important factor, although a degree of direct damage to the smooth muscle also occurs.
Deterioration of anorectal function after radiotherapy has also been attributed to direct radiation damage to the anal sphincters. Decrease in resting and/or squeeze anal pressures has been demonstrated as an indirect evidence of this injury in many studies [22, 23]. Accordingly, DaSilva et al. [24] studied the internal anal sphincter from abdominoperineal resection specimens of 18 patients, 12 of who had preoperative chemoradiation for rectal cancer. These authors found increased fibrosis and nerve fiber density of the internal anal sphincter in specimens obtained from patients with prior chemoradiation.
In addition to the direct injury of the sphincters, it has been demonstrated that radiotherapy can cause lumbar plexopathy in up to 6 % of patients after short course radiotheraphy for rectal cancer [20] (see Chap. 14 for additional information about lumbosacral plexopathy).
Radiotherapy for distal rectal cancer can also damage the pudendal nerve as it courses within the field of irradiation [25]. In a study of 66 patients with resectable rectal cancers who received 45 Gy doses over 5 weeks plus 5-fluorouracil (5-FU) (350 mg/m2/day) and leucovorin (20 mg/m2/day) concurrently on days 1–5 and 29–33 [23], a significant deterioration in incontinence score and anal squeeze pressures occurred while resting pressures remained unchanged. In this study, 26 patients who had rectal cancer with a distal margin within 6 cm of the anal verge had the anus included in the field of radiotherapy, whereas patients with more proximal tumors (6–12 cm from the anal verge; N = 40) had shielding of the anus during radiotherapy. Both groups had similar functional results. In total, 18 patients (27 %) developed unilateral or bilateral pudendal neuropathy after chemoradiation. Another four patients with unilateral prolonged pudendal latencies at baseline, developed prolonged terminal motor latencies of the contralateral pudendal nerve as well. These patients had worsened incontinence scores and squeeze pressures. These occurred independent of tumor response to chemoradiation. Pudendal neuropathy therefore, may explain the decrease in squeeze pressures following chemoradiation found in many studies and thus, contribute to poor functional results after restorative proctectomy for rectal cancer.
Not all studies of neoadjuvant chemoradiation show a high degree of fecal continence in rectal cancer patients. Pietsch et al. [26] compared clinical and manometric parameters before and after surgery (evaluations at 3–6 months and 6–12 months) in 27 patients who were treated by surgery alone and 12 patients who received neoadjuvant chemoradiation (5-FU, CPT-11, and 45 + 5.4 Gy boost). Preoperatively, none of the patients had symptoms of fecal incontinence and manometric parameters were normal. Postoperatively, fecal continence was impaired in both groups, but no significant difference was found between patients with or without chemoradiation. Similarly, anorectal manometry parameters revealed an impairment of anorectal function after low anterior resection regardless of treatment regime. The authors concluded that impairment of anal continence after low anterior resection is determined by the surgical procedure only and not aggravated by neoadjuvant chemoradiation. These results are in accordance with other reports in the literature [27–29]. In addition to the limited number of patients studied, the timing of postoperative measurements is a limiting factor in these studies and may explain discrepancy among authors [30–35]. With recent ongoing advances in neoadjuvant therapy regimes, a tendency to more favorable postoperative functional results can be expected, however results are still conflicting as seen in Table 10.1 [36–40].
Table 10.1
Effects of neoadjuvant radiotherapy on anorectal function: results of literature
Author | Year | Preoperative Regimen | n | Impairment of anorectal function | Follow- up | Manometric evaluation |
|---|---|---|---|---|---|---|
Dahlberg et al. [30] | 1998 | Short term | 171 | Yes | 5 years | No |
van Duijvendijk et al. [32] | 2002 | Short term | 34 | Yes | 1 year | Yes |
Welsh et al. [33] | 2003 | Short term | 124 | Yes | 3 years | No |
Peeters et al. [34] | 2005 | Short term | 597 | Yes | 5 years | No |
Pollack et al. [35] | 2006 | Short term | 21 | Yes | 14 years | Yes |
Birnbaum et al. [27] | 1994 | Conventional | 10 | No | 3 years | Yes |
Gervaz et al. [31] | 2001 | Conventional | 45 | Yes | 2 years | No |
Denhi et al. [36] | 2002 | Conventional | 28 | Yes (Partially) | 1 year | No |
Nathanson et al. [28] | 2003 | Conventional | 109 | No | 5 years | No |
Ammann et al. [25] | 2003 | Conventional | 28 | Yes | 1 year | Yes |
Saito et al. [29] | 2004 | Conventional | 20 | No | 1 year | Yes |
Pietsch et al. [26] | 2007 | Conventional | 12 | No | 6 months | Yes |
Coco et al. [37] | 2007 | Conventional | 100 | Yes | 1 year | No |
Canda et al. [38] | 2010 | Conventional | 31 | Yes | 1 year | Yes |
Denost et al. [39] | 2011 | Conventional | 51 | Yes | 5 years | No |
In a recent systematic review and meta-analysis, Loos et al. [40]analyzed the long-term functional results of patients with rectal cancer undergoing preoperative chemoradiation. The methodological quality of the 25 studies identified was poor. The majority of studies reported higher rates of anorectal dysfunction (14/18 studies) and male sexual dysfunction (9/10 studies). A limited number of studies examined female sexual dysfunction (4 studies). Meta-analysis revealed that the symptom of fecal incontinence occurred significantly more often in irradiated patients and manometric parameters of resting and maximum squeeze pressures were significantly decreased after preoperative radiochemotherapy. The authors concluded that although the data on long-term functional outcome are limited, current evidence demonstrates that preoperative radiochemotherapy negatively affects anorectal function after rectal resection (using total mesorectal excision technique) and patients need be informed about this potential harm of treatment. A multitude of additional factors, including the inherent effect of age or comorbidities such as diabetes mellitus in the sphincter mechanism and the effects of many conditions that alter stool consistency, can participate in the complex etiology of fecal incontinence.
Since survival from rectal cancer has improved substantially over the last two decades, therefore there is also increasing concern about the long-term consequences of the current therapeutic regimens on quality of life. Knowles et al. [41] recently studied the prevalence of pelvic dysfunction and the impact on quality of life after curative colorectal cancer surgery with or without radiotherapy. Patients responded three validated questionnaires; the median time interval following treatment was of 4.4 years. In the 138 patients studied that had undergone treatment for rectal cancer, the following defecatory complaints were found: incontinence of gas: 32 %, fecal leakage: 16 %, wear of pad: 17 %, and incomplete evacuation: 31 %. Preoperative radiotherapy and low level of anastomosis (< 6.0 cm) were associated with increased defecatory problems. New models of comprehensive evaluation and interventions in patients who are at risk of experiencing these late adverse effects should be designed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree