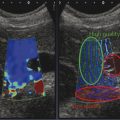
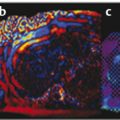
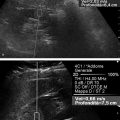
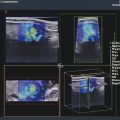
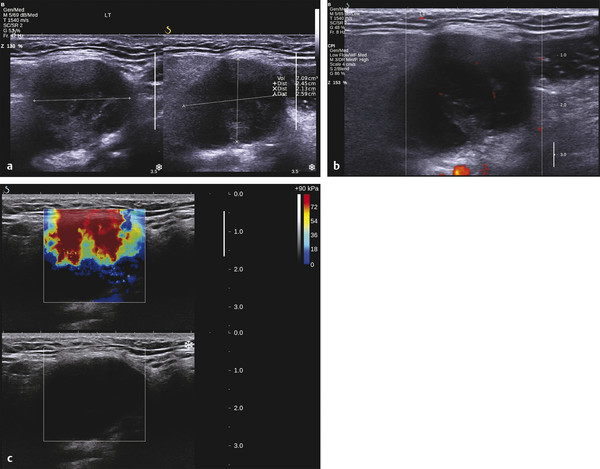
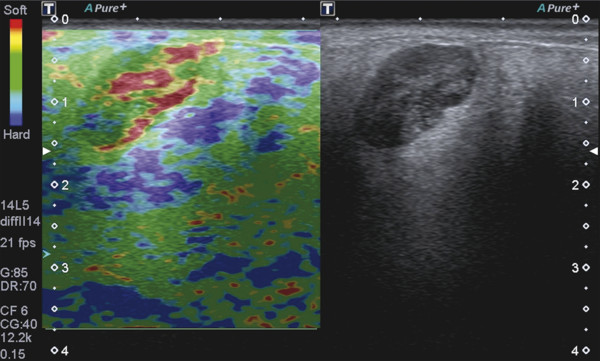
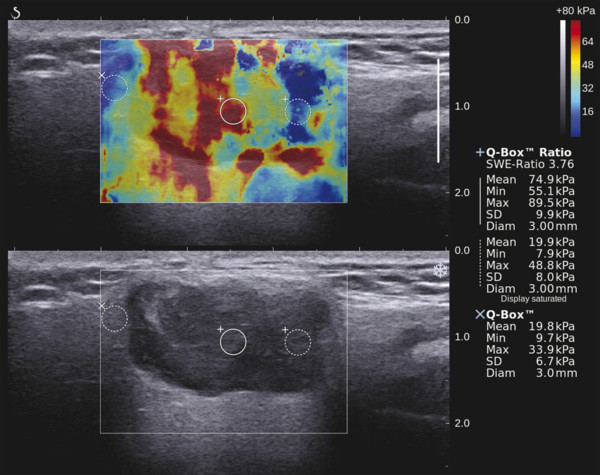
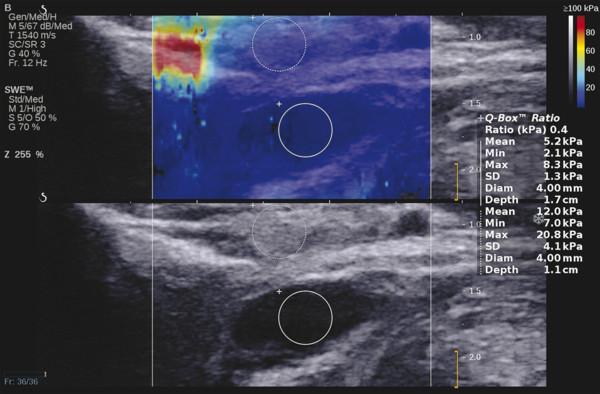
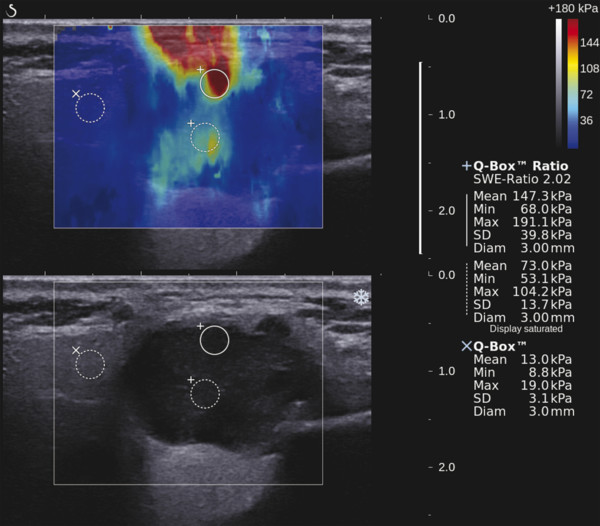
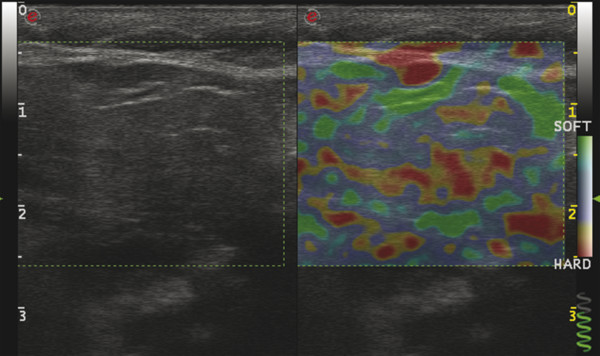
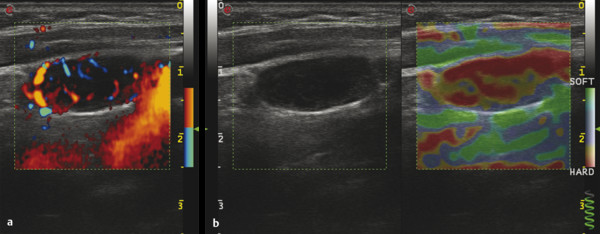
Several criteria for distinguishing between benign and malignant lesions are well described in ultrasound examinations of parotid tumors (▶ Fig. 11.1a). Benign tumors usually have been reported to present with regular and sharp margins, a homogeneous hypoechoic structure, and defined vessel distribution, whereas malignancies lack these characteristics and are irregular, heterogeneous, and diffusely perfused (▶ Fig. 11.1b).2 Unfortunately, features monitored on B-mode and Doppler imaging are inadequate for differentiating benign from malignant salivary gland lesions,3 since their appearance overlaps extensively within the heterogeneous group of benign and malignant salivary gland lesions. In particular, many benign tumors, above all pleomorphic adenomas, can appear irregularly shaped, with heterogeneous echo structure, and therefore cannot be distinguished from malignancies. For these reasons, even skilled ultrasound operators are not so accurate in differentiating benign from malignant lesions. The introduction of ultrasound contrast agents opened up the possibility of more accurate classification of these lesions. Contrast agents demonstrated some differences in flow kinetics between pleomorphic adenomas and Warthin’s tumors;4 however, results are not unequivocal and contrast-enhanced ultrasound (CEUS) does not appear feasible yet in routine clinical work. Fig. 11.1 Pleomorphic adenoma of the parotid gland. (a) B-mode ultrasound shows a heterogeneous appearance with regular, lobulated margins. (b) Power Doppler ultrasound shows little evidence of vascularization. (c) Shear wave elastography shows a heterogeneous stiff appearance. More recently, elastography, an innovative tool for evaluating the stiffness/elasticity characteristics of different tissues and lesions, has proven to be useful for differentiating between distinct types of salivary gland lesions (▶ Fig. 11.1c). A second important clinical scenario is represented by salivary dysfunction as a complication of different possible causes— Sjögren syndrome (SS), radiotherapy, fibrosis, and repeated acute sialadenitis— producing chronic sialadenitis. For all these situations, ultrasound of the major salivary glands is the most attractive imaging option, as it is a noninvasive, inexpensive, and nonirradiating investigation. According to available data, ultrasound yields quite definitive information about the morphological changes of salivary glands. In recent years, also, Doppler imaging and CEUS have been used to evaluate the vascular anatomy of the salivary glands and to analyze the physiological changes in blood flow that occur during salivary stimulation in the diseased glands. Also in this case, elastography, providing information about the stiffness increase related to fibrotic changes of the glands, could represent an important additional tool, useful to evaluate disease progression and to assess the effect of the therapy. Bhatia et al5 examined 6 malignant and 59 benign lesions of salivary glands using longitudinal compression and a qualitative 4-point scoring method. They concluded that pleomorphic adenomas were significantly stiffer than Warthin’s tumors (▶ Fig. 11.2); however, they thought that strain elastography (SE) might have a limited role in distinguishing benign salivary gland masses from malignant lesions because malignant tumors are prevalently stiff. Dumitriu et al6 reported preliminary findings using a different qualitative classification whereby the mass was considered malignant if the stiffness in the mass was greater than 50% of the total mass, whereas the mass was considered benign if the stiffness in the mass was less than 50% of the total mass. In another study with 74 salivary gland masses, Dumitriu et al showed that the difference in elastographic score was statistically significant in discriminating benign from malignant tumors, but the overlap between pleomorphic adenomas and malignant tumors and between pleomorphic adenomas and Warthin’s tumors limits the role of the technique in clinical practice.7 Later, the same authors adopted the 4-point scoring method to evaluate 18 malignant and 56 benign salivary gland tumors using both longitudinal and transverse scans. They determined that the differences in the elastographic scores between benign and malignant tumors overall were statistically significant.7 However, again they pointed out that the differences between malignant tumors and pleomorphic adenomas were not statistically significant. The mean score for pleomorphic adenomas (2.58 ± 0.87) was higher than that for Warthin’s tumors (2.15 ± 0.80), but these differences were not statistically significant. Yerli et al3 examined 36 salivary lesions (28 benign and 8 malignant). They found a score of 3 or 4 in 7 of 11 pleomorphic adenomas, and a score 1 or 2 in 9 of 11 Warthin’s tumors. The authors explained the major stiffness of pleomorphic adenomas on a histopathological basis. Celebi and Mahmutoglu8 examined 81 salivary lesions (49 benign and 32 malignant) in 75 patients adopting the 4-point scoring method. Elastography correctly diagnosed 30 of 49 benign tumors (sensitivity, 61.2%) and 19 of 32 malignant tumors (specificity, 59.4%). The authors found the diagnostic value of elastography to evaluate pleomorphic adenomas, Warthin’s tumors, and high-grade tumors was low, but the diagnostic rates that resulted for low-grade tumors such as mucoepidermoid carcinoma, acinic cell carcinoma, and metastases of basal cell carcinoma were better with elastography. Fig. 11.2 Warthin’s tumor at strain elastography. The benign lesion appears mostly soft. Mansour et al9 examined 33 salivary lesions (29 benign and 4 malignant) in 32 patients using B-mode US, color Doppler, strain elastography (SE), and ARFI quantification. The authors did not visualize any significant difference between pleomorphic adenomas and Warthin’s tumors at strain elastography (SE) evaluation. Klintworth et al2 investigated B-mode and elastographic criteria to differentiate benign from malignant parotid tumors and tried to define elastographic patterns characteristic for pleomorphic adenomas and Warthin’s tumors. In the analysis of 57 patients with parotid gland tumors, the authors stated that different patterns were observed for particular histological subtypes of parotid gland tumors. They concluded that elastography can improve the diagnostic performance of ultrasound and can help the differentiation of benign from malignant parotid tumors. Klintworth et al defined the elastographic garland sign pattern. A reticular distribution of stiff tissue within the whole tumor, the garland sign was seen more frequently in malignant parotid tumors (▶ Fig. 11.3). Pleomorphic adenomas showed an elastographic dense core sign, a central zone of very stiff tissue with softer tissue in the periphery. Warthin’s tumors showed an elastographic half-half sign, with a stiff area located in the superficial half of the lesion while the deeper part had a softer appearance. Parotid cysts showed an elastographic bull’s eye sign, a very soft, elliptic area in the center of a lesion. All the described patterns resulted in statistically significant diagnostic results. Fig. 11.3 Garland sign is a diagnostic artifact that can be seen as a reticular distribution of stiff tissue within the whole tumor in the elastogram. In such cases quantitative evaluation is less useful than the visual display of a reticular distribution of stiff tissue for lesion characterization. Fewer studies on the evaluation of focal salivary gland lesions with shear wave elastography (SWE) have been published to date. Arda et al10 reported the normal values of 10.38 ± 3.5 kPa for the parotid glands and 10.92 ± 3.1 kPa for the submandibular glands (▶ Fig. 11.4) in 127 normal subjects. More recently Mantsopoulos et al11 reported the mean SWE velocities in meters per second in 25 consecutive healthy subject to be 1.854 m/s for parotid glands and 1.932 m/s for the submandibular glands. Bhatia et al12 in their study of 60 lesions (55 benign and 5 malignant) presented practical aspects and potential pitfalls of the approach in diagnosing focal salivary gland lesions; the authors concluded that the potential role of elastography at this site is unclear and unsuitable for ruling out malignancy (▶ Fig. 11.5). Similarly, Westerland and Howlett13 in a review paper found the initial results to be disappointing. Wierzbicka et al14 in their study of 43 patients (33 benign and 10 malignant) found a statistically significant difference between benign and malignant tumor mean elasticity, measured objectively and quantitatively in kilopascals. Moreover, qualitatively malignant tumors visually presented more extensive areas of stiffness. However, the very high standard deviation and range of the results partially confirmed a more skeptical point of view. Olgum et al15 have tried to suggested that the relative proportions of stromal to cellular components of pleomorphic adenomas have an effect on the stiffness values determined by SWE, noting relatively low kilopascal values with a low stromal component of the tumor and, conversely, high kilopascal values with an increased stromal component. Fig. 11.4 Sublingual gland with a small cyst at shear wave elastography. The quantitative evaluation shows a mean value of 12 kPa, in line with the results of Arda and colleagues10 (note that the cyst has a lower mean elasticity value). Fig. 11.5 Adenoma with malignant transformation at shear wave elastography quantitative evaluation. After having discussed the use of elastography to characterize salivary gland’ focal lesions, we will focus now on the evaluation of diffuse pathologies. Focal and diffuse pathologies are investigated equally in other organs (e.g., the liver), while in the salivary glands diffuse pathologies are less frequently and more poorly investigated as compared to focal lesions. Elastography is generally applied to chronic diffuse pathologies (▶ Fig. 11.6) since acute ones (e.g., acute inflammation) are easily evaluated combining B-mode and Doppler information.16. The application of elastography has therefore a limited value for acute diffuse pathologies. Fig. 11.6 Sjögren sialoadenitis of the parotid gland at strain elastography. The strain evaluation demonstrates an inhomogeneous increase of the stiffness values of the entire gland. Almost no studies on diffuse pathologies of salivary glands have been performed with SE, the main limitation probably being the fact that, in order to obtain quantitative data, a reference tissue (e.g., normal parenchyma or subcutaneous tissue) is needed. However, in a diffuse pathology of the salivary glands, normal parenchyma is not present and subcutaneous tissues are generally very thin, thus making reference ROI placements almost impossible. In addition, color-coded maps are useless in the follow-up of a chronic diffuse pathology in which only subtle elasticity modifications are expected. The sum of these factors has probably generated the paucity of research concerning the use of strain imaging in diffuse pathologies of the salivary glands. Badea and colleagues17 reported a case in which the fractal value difference between healthy and pathologic submandibular glands increases the most when real-time SE is used, while a lower value is reported when using SWE. As a result, the article thus suggests fractal analysis as a tool to quantify color-coded maps of SE imaging in order to obtain quantitative data (the fractal value). The application of fractal analysis to SE requires a thorough process of validation, since the literature reports results from only a single case. As already stated, almost all the research performed to evaluate the use of elastography for diffuse pathologies of the salivary glands employs SWE to quantitatively assess parenchymal stiffness. There are two main lines of research: the first one is more developed and regards postradiation gland evaluation, while the second one concerns chronic inflammatory diseases (e.g., Sjögren syndrome). Postradiation changes in salivary glands lead to different degrees of feeding impairment thus heavily influencing the quality of life. Badea and colleagues16 evaluated the submandibular glands of 18 patients who had undergone radiation therapy. The results were compared to the values of a control group composed of healthy volunteers. The elasticity mean value in pathologic patients was 2.13 ± 0.52 m/s versus an elasticity mean value of 1.82 ± 0.41 m/s in the control group. This difference is statistically significant (p < 0.05) and it demonstrates the capability of SWE to differentiate between a gland subjected to radiotherapy and a normal one. A salivation test (a clinical test to estimate gland functioning) was additionally performed; however, the results do not correlate to the elastographic values. A similar study was conducted by Kaluzny and colleagues.18 Fifty-two patients who had undergone radiotherapy for head and neck cancer were evaluated, and the results were compared to a control group composed of healthy volunteers. The parotid mean elasticity value was 41.7 kPa for pathologic glands (versus 26.03 kPa for healthy controls; p = 0.0018) and was 37.6 kPa for pathologic submandibular glands (versus 22.4 kPa for healthy controls; p = 0.0005). The results were also correlated to the intensity of symptoms, which were evaluated by using a clinical index; however, no statistically significant correlation was found. Measures were repeated several times after radiotherapy but the impact of time on elasticity remains unclear. The results from these studies are promising since SWE–unlike B-mode sonography–can differentiate between post-radiation glands and nonirradiated ones. However, further studies are needed to fully investigate the correlation between the severity of symptoms and the elasticity value, and to explore variations related to treatments addressed to reduce post-radiation salivary impairment. The other major line of research in the field of diffuse salivary glands pathologies is chronic inflammatory diseases. Wierzbicka and colleagues19 recently published their results on 78 patients with a large number of different chronic inflammatory pathologies ranging from Sjögren syndrome (20 patients) to Stensen’s duct stenosis (15 patients). In all subgroups mean elasticity values are significantly higher than mean elasticity values of healthy controls (e.g., 111 kPa for patients with Sjögren syndrome versus 24 kPa for healthy patients). Unlike in postradiotherapy patients, in this study, elasticity correlates with the severity of symptoms. For patients with sialolithiasis, similar results were obtained by Zengel and colleagues20 on a smaller but more homogeneous group of 30 patients. The average elasticity value on the afflicted side was 3.20 ± 1.04 m/s with a highly significant difference in comparison to the healthy side of the patient (1.90 ± 0.45 m/s; p < 1.87451E-17). These results suggest that SWE has the potential to become a valuable diagnostic tool for chronic inflammatory diseases of the salivary glands. However, both the authors point out that more studies are needed to evaluate the temporal evolution of stiffness, and the correlation between elasticity and response to therapy. Elastography has shown the ability to improve ultrasound evaluation over B-mode US alone in the detection of diffuse pathologies of the salivary glands. The future steps are to further explore the potential of correlating elasticity value with clinical indexes, functional tests, response to therapy, and evolution of structural changes over time. After having discussed the techniques, tips and tricks concerning the study of salivary glands, we will now focus on artifacts and pitfalls. We will not discuss the general types of artifacts that are common to all anatomical structures and which have already been covered in Chapters 1 to 3. We will focus only on artifacts that are specific or particularly relevant to salivary glands. These artifacts can be divided into two major groups: artifacts related to the position of salivary glands and to their surrounding structure, and artifacts related to histological features (both related to the healthy parenchyma and to pathologic nodules). These artifacts can be found both in SE and SWE, although they are more prominent in SE. SE is greatly affected by mistracking artifacts related to the sliding of the transducer.2 The sliding is caused by the anatomy of the area, which is slippery and presents only a few plane surfaces suitable to hold the probe firmly. Furthermore, artery pulsation and breathing tend to cause a lateral sliding of the probe while compressing–releasing. This out-of-plane compression–release leads to low-quality elastograms affected by mistracking artifacts.12,13 On the other hand precompression artifacts can be as negative as mistracking artifacts: in order to hold the probe in the same position, the operator must apply an excessive pressure on the transducer. This pressure compresses the parenchyma and the lesion before the elastography is performed thus modifying the elasticity of tissues. For these reasons compression–release movements on this area must be extremely gentle and, at the same time, very precise in order to avoid both out-of-plane and precompression artifacts. SWE has the advantage of being free from compression–release artifacts.10 Nonetheless, other artifacts related to salivary gland position and surrounding structures affect both techniques in the same way. These artifacts are caused by three factors: the proximity to the skin,5 the proximity to osseous planes such as the ramus of the mandible12 and the temporal bone,3 and the presence of convex cutaneous surfaces. All these factors contribute, with different mechanisms, to generate local inhomogeneity of the stress thus hiding the real elasticity of the tissue. The proximity to the skin, which presents in this area only a small amount of subcutaneous tissue, generates a hard stripe on the superficial margin of the nodule within the gland (and less frequently on the superficial margin of the gland as well). This linear horizontal artifact can also present a vertical component12 crossing the nodule and completely (for smaller nodules) or partially hiding their true elasticity. This artifact is caused both by a local stress concentration and by the presence of an interface between tissues showing a different elasticity.21 It is easy to rule out its artifactual nature since no inhomogeneity can be seen on B-Mode images. At deeper levels, the bone cortex (e.g., the ramus of the mandible and the temporal bone) can reflect waves thus determining focal inhomogeneity of the stress and harder artifactual areas.3 Lastly, the convexity of the cutaneous surface (both related to the anatomical site and/or big-size nodules) can focally concentrate the stress in the central part of the lesion that will therefore appear hard. This artifact can completely alter the interpretation of the elastogram. Bhatia et al also reported such a central hard area in a lesion that proved to be a lipoma.12 To neutralize this artifact, a practical tip is using a considerable amount of gel in order to artificially smooth the probe-skin interface without losing the acoustic window on the sides of the probe. The parenchyma of healthy salivary glands is generally stiffer than that of other superficial organs (e.g., breast) thus leading to a quicker reduction of signal intensity in the deeper areas of the glands.3 This phenomenon does not generate proper artifacts; however, it limits the possibility to discriminate the elasticity of deeper lesions, thus representing a major limitation especially in the study of lesions of the deep lobe of the parotid gland.7 Salivary glands lesions (similar to thyroid lesions) often present with cystic components that are more rarely seen in other organs (e.g., the liver). Salivary glands, unlike the thyroid gland, can also show a dilatation of the ducts. All these cystic and pseudocystic aspects of both parenchyma and nodule can lead can lead to inaccurate measurement, with an artificial hardening of the surrounding tissues,21 related to the inability of elastography to measure the elasticity of the fluid components. These artifacts can be avoided by measuring the elasticity in the solid portion of the lesion most distant from the cystic portion. On the other hand, some authors suggest exploiting this artifact in order to find hidden cystic portions of salivary gland lesions. These lesions are often very fibrous and thus very hypoechoic on B-mode ultrasound; this makes it extremely hard to identify these small cystic areas.6 However, their typical color stratification pattern is easy to detect with the aid of elastography,14 thus revealing hidden cystic areas and giving the operator more information to identify the nature of the lesion. Lymph node evaluation in medicine is generally aimed at finding metastases from solid tumors22 or assessing primary involvement from hematologic diseases. Differential diagnosis between neoplastic nodes and non-neoplastic nodes (both normal and with infective or rheumatologic involvement) is another critical diagnostic challenge.23 Before the development of modern imaging, lymph node neoplastic involvement had always been assessed by clinical examination,24 as neoplastic involvement usually increases lymph node stiffness. Metastatic tissue is generally more highly cellulated and thus more stiff than normal lymph node tissue; however, clinical examination has proven to be highly inaccurate in separating normal from metastatic nodes.25 Several modalities have been employed to address lymph node evaluation: US, CT, MRI, and nuclear medicine (fluorodeoxyglucose positron emission tomography [FDG-PET], lymphoscintigraphy).26 Combined modalities (PET/MR and PET/CT) have been employed as well. The introduction of a novel contrast medium such as ultrasmall particles of iron oxide (USPIO) in contrast medium27 or of a new generation of US contrast agent with perflbutane microbubble,28 demonstates that each modality is subject to a constant updates. With all these options available, several criteria have been taken into account: nodal size, shape, site, outline, internal appearance, and behavior after contrast medium administration. However, all these modalities, techniques, and criteria (or combination of them) have been demonstrated to be suboptimal in separating normal from metastatic nodes and for this reason invasive staging approaches are still irreplaceable.29 If we take into account these considerations, ultrasound is frequently advocated as an important instrument in lymph node evaluation (both for superficial and deep lymph nodes, thanks to endoscopic ultrasound [EUS]).29 In fact, US shows sensitivity and specificity close to that of other imaging modalities, and it can also be used to guide biopsies and to target the most probable metastatic site inside the lymph node.30 As already extensively stated, the principle of elastography is simple and effective and can be exploited in order to identify micrometastatic foci (seen as hard areas) thereby realizing an empowerment of US, enhancing prebiopsy evaluation of lymph nodes and thus directly influencing the choice and final outcome of the patient’s treatment. Elastography can be used to assess superficial and deep lymph nodes with a superficial linear probe and an endoscopic ultrasound probe, respectively. Lyshchik et al31 published one of the first clinical studies to evaluate the diagnostic effectiveness of SE by examining 141 lymph nodes, of which 98 were confirmed benign and 43 were confirmed malignant by histopathology. They classified the nodes according to visualization, brightness compared to the neighboring muscles, and the regularity of the outline. Using a strain ratio cutoff value of >1.5, SE demonstrated sensitivity, specificity, and accuracy values of 85%, 98%, and 92%, respectively. According to a meta-analysis of nine SE studies that included 50 to 155 cervical or axillary lymph nodes,32 the pooled sensitivity and specificity values for detecting malignancies were 74% and 90% using an elastographic scale and 88% and 81% using strain ratios, respectively. The 4-point elastographic scale for suspect malignant lymph nodes proposed by Lo et al is simpler and used more widely. In general, metastatic lymph nodes demonstrate higher stiffness (▶ Fig. 11.7) than benign lymph nodes (▶ Fig. 11.8), so elastographic scale scores of 1 and 2 indicate benign lymph nodes, and elastographic scale scores of 3 and 4 indicate malignant lymph nodes.33 Fig. 11.7 Metastatic lymph node. (a) Power Doppler evaluation. (b) Strain elastography evaluation. The node appears mostly hard.
11.2.1 Focal Lesion Differential Diagnosis
Strain Elastography Imaging
Shear Wave Elastography Imaging
11.2.2 Fibrosis: Salivary Gland Diffuse Pathologies
Strain Elastography Imaging
Shear Wave Elastography Imaging
Summary
11.2.3 Artifacts and Pitfalls
Artifacts Related to Gland Position and Surrounding Structures
Artifacts Related to Histological Feature
11.3 Lymph Nodes
11.3.1 Superficial Lymph Nodes: Indications and Clinical Applications
Strain Elastography Imaging
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree