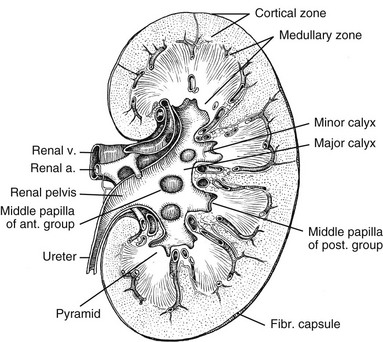
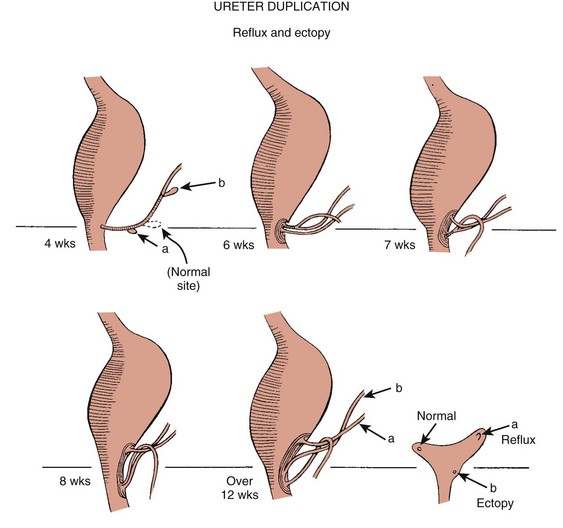
Chapter 111 The urinary system and the genital system are closely associated embryologically and begin to develop during the fourth week of gestation.1,2 Both develop from the intermediate mesoderm along the posterior wall of the abdominal cavity. A longitudinal elevation of the mesoderm, the urogenital ridge, forms on both sides of the abdominal aorta. Part of the urogenital ridge gives rise to the nephrogenic cord, which will form the urinary system, and another part gives rise to the gonadal ridge, which will form the genital system (see Chapter 125).1 The nephrogenic cord gives rise to the mesonephros, which consists of glomeruli and mesonephric tubules. The mesonephric tubules open into the mesonephric duct, which soon opens into the cloaca.1,3 The mesonephros degenerates toward the end of the first trimester, although their tubules persist in males and participate in the formation of the genital system.2 During the fifth week of gestation, the metanephros begins to develop. The definitive kidneys develop from the ureteric bud (metanephric diverticulum), an outgrowth of the mesonephric duct, and the metanephric blastema, which is derived from the nephrogenic cord. The ureteric bud is responsible for the development of the collecting system (ureter, renal pelvis, and calyces).1,2,4 The stalk of the ureteric bud forms the ureter. The ureteric bud grows into the metanephric blastema, where branching leads to the formation of the renal pelvis, major and minor calyces, and the collecting tubules (Figs. 111-1 and 111-2). Nephrons form in the metanephric blastema as the result of induction by the collecting tubules. The embryonic kidneys initially lie in the pelvis. As the abdomen grows, the kidneys “ascend” and rotate 90 degrees. By the ninth week of gestation, the kidneys come in contact with the adrenal glands as they attain their final position. The fetal kidney has a lobulated external contour that will disappear as the nephrons continue to grow. The full complement of glomeruli is present by 36 weeks’ gestation. Figure 111-1 Development of the metanephros, the primordium of the permanent kidney. Congenital anomalies of the urinary tract are common.1,4 Early degeneration of a ureteric bud or involution of the metanephros leads to regression of the metanephric blastema and renal agenesis. If the ureteric bud and metanephric blastema do not join normally, abnormal induction of the blastemal elements is likely to result in a multicystic dysplastic kidney. Bifurcation of the ureteric buds results in partial duplication of the collecting system. When the location of the origin of the ureteric bud is abnormal or if the origin itself is maldeveloped, the potential for vesicoureteral reflux (VUR) or ureteral ectopia exists (Fig. 111-3). Figure 111-3 The ureteric bud and its relationship to the bladder. The drawing shows how ectopia may occur. The bladder arises from the most superior portion of the urogenital sinus.1,2,5 It is initially continuous with the allantois. With constriction of the allantois, the lumen is obliterated, and a thick, fibrous cord, the urachus, remains. The urachus connects the apex of the bladder with the umbilicus. Persistence of the allantoic lumen may result in urachal fistulas, sinuses, or cysts. The ureterovesical junction (UVJ) develops as the distal parts of the mesonephric duct are incorporated into the enlarging bladder. As the mesonephric ducts are absorbed, the ureters come to open separately into the urinary bladder with the orifice moving superolaterally and the distal ureteral segments entering obliquely through the base of the bladder. The middle portion of the urogenital sinus develops into the prostatic urethra in males and the entire urethra in females. The distal portion of the male urethra is derived from a cord of ectoderm that grows from the tip of the glans penis to meet the spongy portion of the urethra derived from the caudal (phallic) portion of the urogenital sinus. Development of the ureters and bladder is complete by the fourth gestational month. The fetus begins to make urine by the ninth week of gestation, and the urine contributes the largest component of the amniotic fluid.6 Oligohydramnios is often a marker of urinary tract abnormalities, and severe oligohydramnios may lead to pulmonary hypoplasia. During the first few days after birth, the neonate has a low glomerular filtration rate (GFR) and low urine output.7 This is especially true of premature infants. The newborn kidney also has a limited ability to concentrate urine and decreased tubular reabsorption of sodium. GFR and urine output increase significantly over the first week of life. This immaturity of renal function is an important factor to keep in mind in the ordering and interpretation of renal imaging studies. For example, the low urine output present on day one of life may mask the presence of hydronephrosis on renal ultrasonography. The renal parenchyma is composed of two distinct regions: (1) the outer cortex, and (2) the inner medulla (Fig. 111-4). Within the cortex are the nephrons. The medulla is composed of 8 to 13 pyramids, which terminate in the renal papillae at the level of the calyces. Two or more pyramids may drain into the same papilla (confluent papilla), and two or more papillae may drain into a single calyx (compound calyx). A column of Bertin represents the renal cortex extending centrally between the pyramids (e-Fig. 111-5), most often occurring at the junction of the middle and upper groups of calyces or between the two central renal hypoechoic complexes of a duplex kidney.8,9 e-Figure 111-5 Column of Bertin. Multiple renal lobules fuse to form the kidney. The junction of these lobules sometimes persists and may be seen as a scalloping of the cortical border (Fig. 111-6). The junctional parenchymal defect is similarly derived from a variation in the fusion of the fetal renunculi or lobules. It appears as a thick, triangular, echogenic notch in the anterosuperior or posteroinferior aspect of the kidney (more often on the right) and mimics a cortical scar. The junctional parenchymal defect may be connected to the renal hilum by an echogenic line called the inter-renuncular septum (Fig. 111-7). The left kidney is apt to be more triangular in shape, with a distinct bulge along its lateral aspect, the so-called dromedary kidney (Fig. 111-8).10–13 Figure 111-6 Fetal lobulations. Figure 111-7 Junctional parenchymal defects. Figure 111-8 Normal neonatal kidney. In newborns and infants, the kidneys have a larger medullary and a smaller cortical volume than in later life. On ultrasonography, the neonatal renal cortex is moderately hyperechoic, close to the echogenicity of adjacent liver and spleen. In newborn and premature infants, the cortical echogenicity may actually be greater than that of the liver (see Fig. 111-8). The pyramids are relatively hypoechoic. Between ages 6 months and 2 years, the echogenicity of the medulla and cortex resembles that of adult kidneys.14–17 The kidney is usually supplied by a single artery arising from the aorta. After the renal artery enters the renal hilum, posterior and slightly superior to the renal vein, it divides into anterior and posterior branches, which, in turn, generally divide into superior and inferior branches (e-Fig. 111-9). Variations exist at all levels. About 20% to 30% of kidneys have a second or accessory renal artery, which also arises from the aorta. The normal renal vein lies anterior and slightly inferior to the renal artery. The left renal vein is longer than the right, courses anterior to the aorta, and receives the ipsilateral suprarenal and gonadal veins before entering the inferior vena cava.8,9,18 e-Figure 111-9 Normal renal arterial anatomy. Renal length is the most commonly measured parameter and has been correlated to age, body height and weight, and height of the first three or four lumbar vertebral bodies. Standards for renal size have been developed for ultrasonography (e-Fig. 111-10). The left kidney may be slightly longer than the right. Kidneys with complete or partial collecting system duplication are longer than normal kidneys. The width of the kidney is approximately 50% of its length and is relatively thicker in neonates than in older children. Cortical thickness of the upper renal pole is normally slightly thicker than the lower pole, and the renal cortex is slightly thinner in the center of the kidney. Extra cortical tissue may be noted about the renal hilum and may impinge from above or below on the renal pelvis (suprahilar or infrahilar bulge, or hilar lips).19–25 e-Figure 111-10 Kidney length in centimeters. The renal pelvis varies in size from a small, poorly defined sac to a large, boxlike structure. The pelvis may lie entirely within (intrarenal) or almost beyond (extrarenal) the renal sinus. The configuration of the pelvocalyceal system is quite variable. In most kidneys, the pelvis branches into two major infundibula (or major calyces). The inferior infundibulum is commonly broad and short and is connected with a larger number of calyces compared with the upper infundibulum. Each kidney has about 8 to 13 calyces. These have a cup-shaped appearance that results from the protrusion of the renal papilla into the calyx. Two or more papillae may enter one calyx (compound calyx). Most calyces are directed laterally and either slightly anteriorly or posteriorly within the kidney.4,6,18,24,26 The ureter is a tubular structure that courses retroperitoneally from the kidney to the bladder and has three major components: (1) the ureteropelvic junction (UPJ) and upper ureter, (2) the midureter, and (3) the lower ureter and the UVJ (including the transmural ureter and the ureteral orifice). The abdominal or upper ureter starts at the UPJ with a smooth tapering from the renal pelvis. The abdominal ureter lies adjacent to the psoas muscle, being crossed by the gonadal vessels before passing behind the iliac vessels. The lower ureter lies along the lateral pelvic wall, coursing downward behind the bladder. As the ureter enters the bladder wall, it takes an oblique course from a superolateral entry point to an inferomedial ostium and eventually opens into the bladder. Its course through the bladder wall may be seen (called the plica ureterica at cystoscopy) and constitutes the lateral border of the bladder trigone. Three muscular layers constitute the ureteral wall, with a thick outer adventitial covering that contains a rich vascular layer and lymphatic plexus.4,6,27–29 The vascular supply of the ureter is from bladder vessels (inferior vesical artery), gonadal arteries (testicular artery), and the renal artery superiorly. Nerves supplying the ureter follow the same course as that of arteries. Rhythmic peristaltic ureteral contractions transport urine from the renal pelvis into the urinary bladder and occur two to seven times per minute.4,6 The anatomic relationship of the distal ureter to the bladder wall at the UVJ is important in preventing VUR. Normally, the distal ureter inserts into the bladder, courses through the bladder musculature at an oblique angle, and then continues inferomedially and submucosally to its ostia in the lateral corner of the trigone (Fig. 111-11). The UVJ acts as a passive flap valve. Continence is ensured by apposition of the roof and floor of the submucosal tunnel when intravesical pressure increases and is enhanced by the action of the intrinsic local musculature. A normal ureteral tunnel length to ureteral diameter ratio in children is 5 : 1. A ratio of at least 3 : 1 is necessary for UVJ competence.30–34 In the neonate and young child, the dome and body of the bladder are located primarily in the abdomen and anterior, whereas its base is intrapelvic and posterior. Bowel impressions are common and may impress on the intraabdominal bladder when it is filled. This normal appearance should not be confused with an intravesical mass or extrinsic compression by a pelvic mass. A urachal remnant may also be seen at the bladder dome. Normal bladder capacity may be predicted on the basis of weight in infancy and by age for the older child. For infants under 1 year of age, bladder capacity in milliliters is predicted by multiplying the weight in kilograms by 7. The predicted bladder capacity in milliliters of children more than 1 year old is the age in years plus 2 multiplied by 30. Ultrasonography best demonstrates normal bladder wall thickness, which should be no more than 3 mm when distended and 6 mm when collapsed.5,35–43 The female urethra corresponds anatomically to the posterior urethra of the male. Anatomic features of the female urethra include the internal sphincter at the bladder neck, intermuscular incisura, membranous urethra at the level of the urogenital diaphragm or external urethral sphincter, and the fossa navicularis.5,44,45 The components of the normal male urethra include the prostatic or posterior urethra, the membranous urethra, the bulbous urethra, and the penile urethra. Normal anatomic features of the male prostatic urethra include the internal sphincter, the intermuscular incisura, and the verumontanum. The membranous urethra runs through the urogenital diaphragm or the external urethral sphincter, after which come the bulbous urethra, the penile urethra, and the fossa navicularis (e-Fig. 111-12). The intermuscular incisura produces an indentation in the midportion of the posterior urethra at the level of the verumontanum and is caused by an abundance of collagenous tissue at this location (more prominent anteriorly). The verumontanum is a focal elevation in the posterior wall of the prostatic urethra where the paired ejaculatory ducts enter, seen as a small ovoid filling defect in the posterior portion of the prostatic urethra. The plicae colliculae are normal folds that extend from the distal verumontanum to the posterior urethra and may produce circumferential impressions on urethral images.5,44,46–50 e-Figure 111-12 Normal urethra. True renal agenesis is thought to be caused by failure of the ureteric bud to contact the ipsilateral metanephric blastema. Unilateral renal agenesis is present in about 1 : 1000 live births. Children with true unilateral renal agenesis lack the ipsilateral ureter and hemitrigone of the urinary bladder. Those children with an absent kidney but a normally developed bladder and a distal ureter of varying length probably represent the involution of a multicystic dysplastic kidney.36 Associated anomalies include VACTERL association (e-Fig. 111-13), unicornuate uterus, uterus didelphys, and Mayer-Rokitansky-Küster-Hauser syndrome in females, and seminal vesicle cysts, absence of the vas deferens, and cystic dysplasia of the rete testis in males (Box 111-1).36,51–58
Embryology, Anatomy, and Variants of the Genitourinary Tract
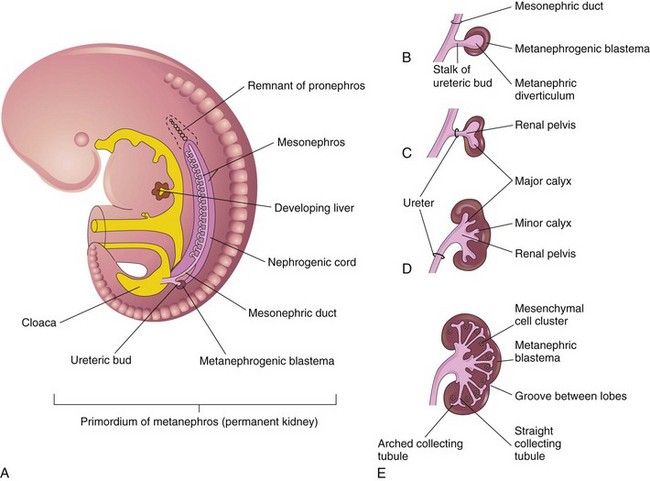
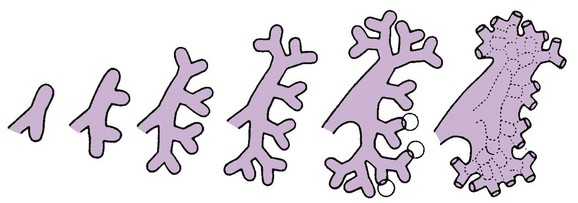
A, Lateral view of a 5-week embryo, showing the primordium of the metanephros. B to E, Successive stages in the development of the metanephric diverticulum or ureteric bud (fifth to eighth weeks). Observe the development of the ureter, renal pelvis, the calices, and the collecting tubules. (From Moore KL, Persaud TVN. The urogenital system. In: Before we are born: essentials of embryology and birth defects. 7th ed. Philadelphia: Saunders Elsevier; 2003.)
Normal Anatomy
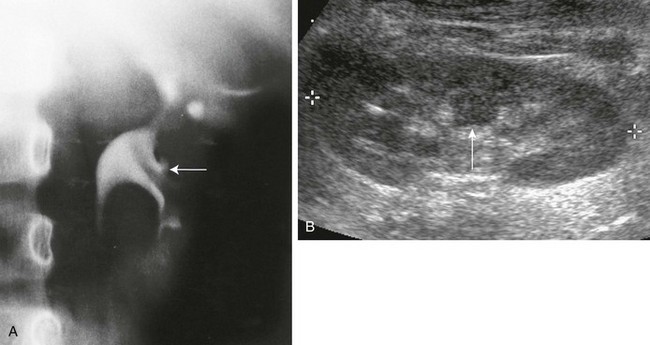
A, Intravenous urography shows that the column of Bertin has produced spreading of the calyces of the left kidney, simulating a renal mass. The centrally located single calyx (arrow) is draining this ectopic tissue. Note also the oblique vascular impression on the lower pole infundibulum (major calyx). B, Longitudinal sonogram in another patient shows a central column of cortical tissue (arrow) extending into the central sinus echoes. (B, Courtesy Brian D. Coley, MD, Cincinnati, OH.)
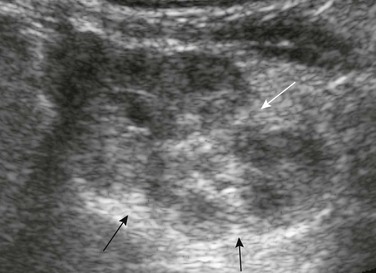
Residual fetal lobulations are responsible for the scalloped border of the kidney (black arrows) on this sagittal sonogram. A prominent junctional cortical defect is also present (white arrow).
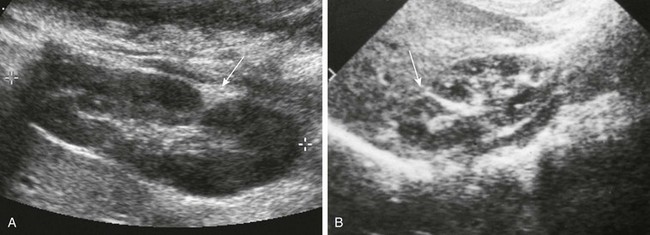
A, Longitudinal prone sonogram shows a fat-filled cleft (arrow) in the posterior kidney. B, Longitudinal supine sonogram in another patient shows a renicular septum (arrow). Both are normal findings, probably related to a demarcation of embryonic reniculi.
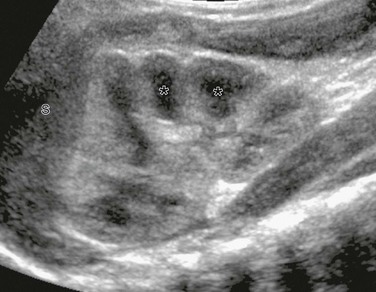
Supine longitudinal sonogram of the left kidney shows the sharp corticomedullary differentiation common in infancy and early childhood. The hypoechoic triangular renal pyramids (asterisks) are surrounded by cortex that is more echogenic than the adjacent spleen (S). The spleen has flattened the upper renal contour and produces the appearance of a dromedary kidney. (Courtesy Brian D. Coley, MD, Cincinnati, OH.)
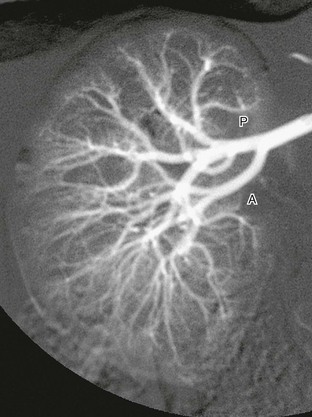
Digital subtraction arteriogram of the right kidney in a 6-month-old shows a single main renal artery dividing into anterior (A) and posterior (P) branches. Normal opacification and distribution of vessels to the level of the arcuate arteries are seen. (Courtesy Brian D. Coley, MD, Cincinnati, OH.)
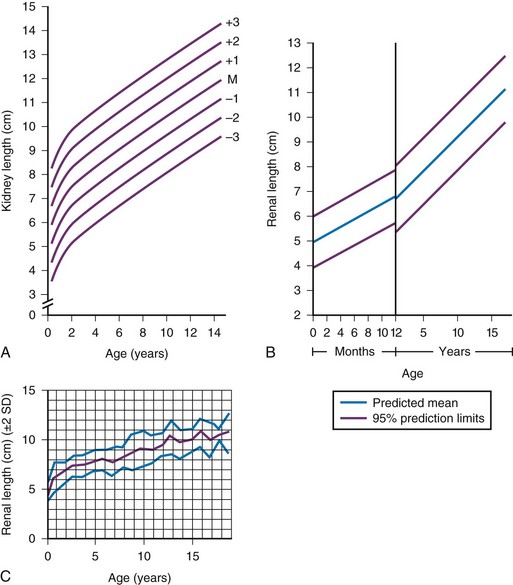
A, Measurements obtained from supine urographic films and correlated with age, including values for 3 standard deviations (SD) above and below the mean (M). The SD is 0.75 cm. B, Ultrasonographic measurements correlated with age; the values are slightly lower than those obtained from urographic films. C, Ultrasonographic measurements correlated with age, including ± 2 SD. (A, Modified from Currarino G, Williams B, Dana K. Kidney length correlated with age: normal values in children. Radiology. 1984;150:703; B, From Han BK, Babcock DS. Sonographic measurements and appearance of normal kidneys in children. AJR Am J Roentgenol. 1985;145:611; C, From Rosenbaum DM, Korngold E, Teele RL. Sonographic assessment of renal length in normal children. AJR Am J Roentgenol. 1984;142:467.)
Pelvocalyceal System
Ureter
Bladder
Urethra
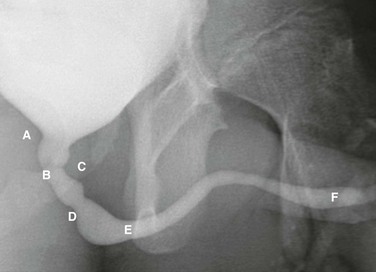
Voiding cystourethrogram in a 15-year-old boy shows a normal urethra. A, External sphincter. B, Site of the verumonatnum. C, Prostatic urethra. D, Membranous urethra. E, Penile urethra. F, Fossa navicularis. (From the Radiographic atlas of the pediatric urethra. Available at www.natiowidechildrens.org.)
Congenital Anatomic Variants and Anomalies
Renal Agenesis (Unilateral and Bilateral)