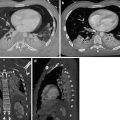
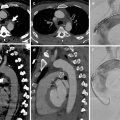
Based on this initial evaluation, the ED physician has to risk-stratify the majority of the remaining patients into one of the three groups of diagnoses of concern (ACS, PE, or AAS) or state that the establishment of a leading diagnosis is not possible (Fig. 1). For ACS and PE, several risk scores have been thoroughly evaluated (the most common ones being the TIMI risk score (Antman et al. 2000) and the GRACE score (Fox et al. 2006) (http://www.gracescore.org/website/WebVersion.aspx) for ACS and the PE Wells score (Wells et al. 2000) and have proven to result in improved clinical decision-making, thus facilitating the classification of a patient into a particular category.
1.3 Triage Pathways in Suspected Acute Coronary Syndrome
In ACS patients, ECG findings diagnostic for myocardial ischemia or early biomarkers confirming myocyte damage are present in only a minority of patients with acute chest pain. The remaining clinically stable patients, however, still have possible myocardial ischemia and suspected NSTEMI ACS. It is in this subgroup of patients in which many are admitted and placed into chest pain units for telemetric observation – which is often referred to as “observational pathway.”
However, in most of these patients, symptoms will later be ascribed to nonischemic causes – in these patients admission to an intermediate care unit could have been avoided if appropriate (imaging) test results had clearly ruled out an ischemic cause at the time of presentation. Strategies aiming at an early imaging-based exclusion of a coronary etiology of the presenting symptoms are commonly dubbed “early assessment path.”
1.4 Indications for CCTA in the “Early Assessment Pathway”
Appropriateness of coronary CTA as part of the early assessment pathway will depend on the initial ECG, initial biomarker, and status of symptoms at the time of presentation. Four randomized trials have compared current standard-of-care management with early CCTA in chest pain patients presenting with a low-to-intermediate probability for ACS (Goldstein et al. 2007; Goldstein et al. 2011; Cury et al. 2013; Litt et al. 2012; Hoffmann et al. 2012). These trials have – individually and in aggregate (Hulten et al. 2013) – confirmed the safety and efficacy of an early CCTA approach in this clinical context.
Based on these trial results, in the large proportion of chest pain patients with low-to-intermediate risk for ACS, without ECG changes typical for ischemia and normal initial troponin, CCTA is considered appropriate (Rybicki et al. 2016).
In patients with initial ECG changes suggesting ischemia (other than ST-segment elevation) and positive initial biomarkers (positive initial diagnosis of NSTEMI/ACS), coronary CTA is not considered appropriate since in these patients, early revascularization may be associated with more favorable outcomes (Roffi et al. 2015).
In European countries, high-sensitivity troponin (hsTrop) assays have recently become widely available. Due to a lack of FDA approval, these are not (yet) available in the United States, however. In patients presenting with chest pain, in whom early hsTrop values are negative and who have a TIMI risk score of 0, no further testing might be considered – however, even in this extremely low-risk setting, coronary CTA is considered appropriate (Rybicki et al. 2016).
In several clinical scenarios, the diagnosis NSTEMI/ACS would be equivocal: either because clinical symptoms resolved hours before (<3 h) and the patient is asymptomatic at the time of presentation to the ED or because the initial troponin is equivocal or elevated without additional evidence of ACS. In these scenarios, coronary CTA is considered appropriate for early assessment of possible ACS (Rybicki et al. 2016).
1.5 Indications for Coronary CTA in the “Observational Pathway”
Several clinical scenarios exist after a patient is admitted in the context of an observational pathway for which recommendations for the use of coronary CTA exist. In the first scenario, any ECG and/or serial troponin unequivocally confirms NSTEMI or ACS. In this context of troponin-positive NSTEMI or ACS, several randomized trials have shown a more favorable outcome of an early invasive strategy, i.e., early revascularization. Coronary CTA is not considered appropriate in these patients unless there is significant comorbidity (particularly renal failure) which would decrease the advantage of an early invasive approach.
On the other hand, serial ECG and biomarkers might be negative or borderline for NSTEMI or ACS. In either case, the use of coronary CTA would be considered appropriate. With negative biomarkers, CTA can be performed on an outpatient basis.
2 CT Imaging and Post-processing Techniques
2.1 General Considerations
With increasing availability of state-of-the-art CT coronary angiography, the role of a calcium-scoring scan only (i.e., non-enhanced, ECG-synchronized scan of the heart) for chest pain evaluation has dwindled. While there were several studies in the late 1990s who investigated this strategy (Georgiou et al. 2001; Laudon et al. 1999; McLaughlin et al. 1999), over the years it has become apparent that the frequency of patients who present with very low calcium scores and yet show highly significant stenoses on coronary CTA or myocardial ischemia on provocative testing is too high to be negligible (Schenker et al. 2008). Furthermore, since many chest pain patients present with significant amounts of calcium, (CT) angiographic workup would be necessary in the majority of patients.
The acquisition of an unenhanced calcium-scoring scan prior to CCTA is controversial. It has been demonstrated that in patients presenting with acute chest pain, there is no incremental prognostic information gained from an independent prior calcium-scoring study (Chang et al. 2011).
At our institution, we perform an initial unenhanced CT scan for calcium scoring in all ACS patients who are over 45 years of age. To keep additional effective dose at the minimum, we choose a high-pitch acquisition protocol on a dual-source CT for this unenhanced scan regardless of the patients’ heart rate and rhythm intending to scan the heart during diastole (we usually do not repeat the scan if there are motion artifacts). In our experience, this has several advantages, both regarding the diagnostic information obtained and familiarizing the patient with the breath holding and table movement procedure. It facilitates the customization of the scan range and thus contributes to dose reduction. Diagnostically, the calcium score will provide a first estimate of the amount of coronary atherosclerosis. It supports the correct interpretation of the subsequent CT angiography scan since it is unambiguous for calcification (Fig. 2). At very high calcium scores, subsequent CT angiography might be waived due to the high probability of not contributing more diagnostic information, although in appropriately selected patients, this only leads to the exclusion of very few (Chang et al.
2011). Furthermore, in patients in whom acute pathologies of the aorta also cannot be excluded, we perform an unenhanced, ECG-triggered scan of thorax covering the entire thoracic aorta intending to scan the heart at diastole. This allows for a 180-mm field-of-view (FoV) reconstruction for the coronary calcium score as well as reconstructions with full FoV for the exclusion of intramural hematoma within the aorta, hemorrhagic pericardial effusion, or hemorrhagic pleural effusions. In our experience, measurements of Hounsfield units within pericardial effusions tend to be the most accurate in unenhanced scans.

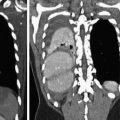
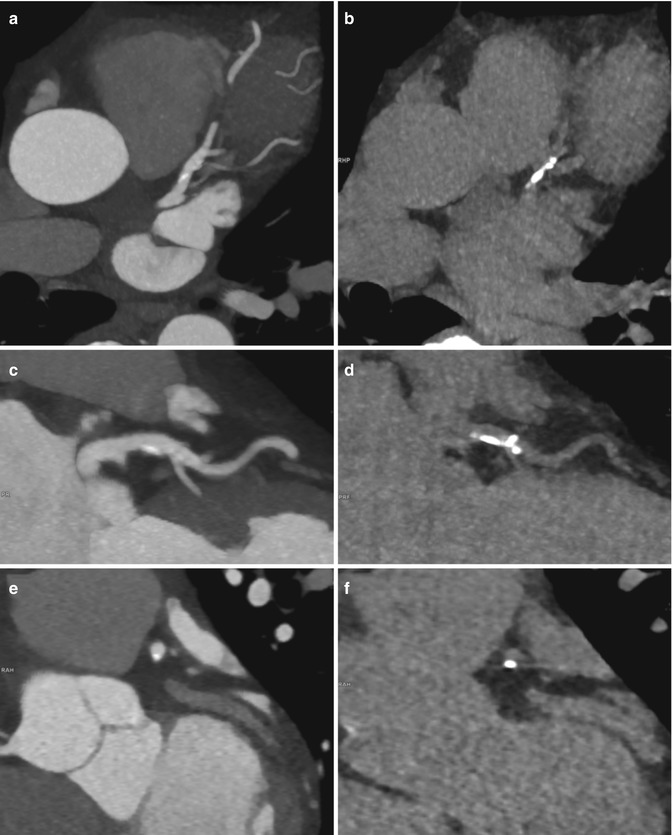
Fig. 2
Comparison of a contrast-enhanced CT-angiography series (a, c, f, 90 kVp) and non-enhanced series (b, d, f, 120 kVp) in a 52 year-old patient presenting with atypical chest pain showing a calcified plaque in the proximal LAD in corresponding 5 mm maximum intensity projection (MIP) reformations. This example illustrates how calcifications are better delineated on non-contrast series
2.2 CCTA Protocol
Besides the usual relative and absolute contraindications for the administration of CT contrast material (impaired renal function, severe allergy to iodinated contrast material, manifest hyperthyroidism and pregnancy), specifically complicating factors for coronary CTA are irregular heart rhythms (atrial fibrillation, frequent extrasystoles) or contraindications to beta-blockers such as moderate-to-severe asthma. However, the vast majority of patients presenting with potential ACS will already have been administered beta-blockers to reduce myocardial oxygen demand prior to referral for coronary CTA (Roffi et al. 2015). In patients with acute chest pain, we perform additional rate control in close collaboration with the treating physician in hemodynamically stable patients using intravenous metoprolol at 5 mg intervals up to a maximum total dose of 20 mg and a target heart rate of ≤70 bpm.
Prior to contrast administration and also in close collaboration with the referring physician, we also apply sublingual nitrates. Importantly, recent use of phosphodiesterase type 5 inhibitors (i.e., within 24 h for sildenafil and vardenafil and 48 h for tadalafil; used for erectile dysfunction or pulmonary hypertension, critical aortic stenosis, and hypotension) needs to be excluded prior to nitrate administration. We would also avoid nitrates in patients with a history of glaucoma. When beta-blockers have been administered previously, reactive tachycardia to nitrates is not a relevant problem in our experience. Particularly in younger patients, patient education about potentially severe headaches is mandatory.
For coronary CTA in patients with chest pain, we usually perform a test bolus acquisition first to determine optimal scan delay. In our opinion a test bolus strategy has several advantages over bolus triggering: in this patient cohort with a very wide range of ejection fractions, a test bolus approach allows for the most accurate modeling of early contrast dynamics. Furthermore, some patients (particularly with cardiac pacemakers) will suffer from chronic subclavian thrombosis which severely impairs contrast material inflow and can be detected during the test bolus. Finally, many patients will develop reactive tachycardia during contrast administration. When using bolus triggering, the heart rate can increase considerably during contrast administration, whereupon the scan protocol cannot be adjusted to the sudden change in heart rate. A scan protocol that would have been adequate for a slow steady heart rate frequently will result in nondiagnostic image quality at higher heart rates. When working with a test bolus, on the other hand, the administration of contrast material for the test bolus will often precipitate the contrast-induced tachycardia already, so the scan protocol can then be adjusted. Conversely, if the heart rate stays low during and after the test bolus, no sudden increase in heart rate during administration of the main contrast bolus is to be expected.
For optimal image quality with sound opacification of the distal coronary segments, a high iodine delivery rate is mandatory. In nonobese patients, we typically use a triphasic injection protocol with an initial bolus of 60 ml pure contrast material (350 mgI/ml or 400 mgI/ml) at 5.0 ml/s followed by a second phase of diluted contrast material (30 ml of a 50:50 mixture at 5.0 ml/s), followed by a saline chaser of 100 ml at the same injection rate.
The choice of scan protocol will be based on patient age, renal function, heart rate, and available scanner hardware. Naturally, the younger the patient, the higher the effort should be to reduce effective dose. In young patients it seems a reasonable strategy to apply an ultra low-dose (<1 mSv) scan protocol first even when there is a non-negligible risk of producing nondiagnostic image quality due to motion artifacts (Deseive et al. 2015). If image quality turns out to be nondiagnostic, the repeated contrast injection necessary for a second scan using a more complex scan protocol will usually not pose a problem to a younger patient’s kidneys. All patients in whom the initial scan produced diagnostic image quality will have received a scan at extremely low effective dose. Patients who need a repeat scan with a more complex protocol (in the range 5 mSv), however, will only have a small increase in the total effective dose.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree