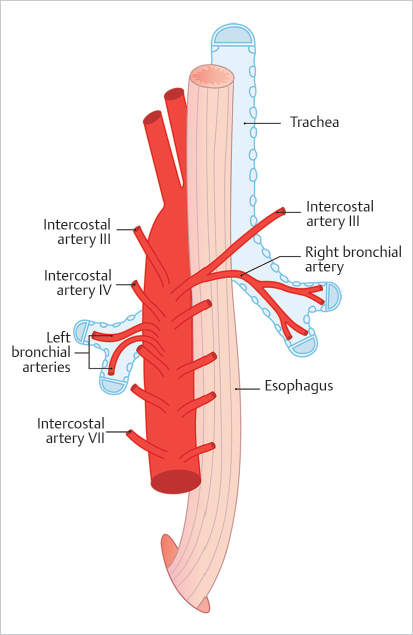
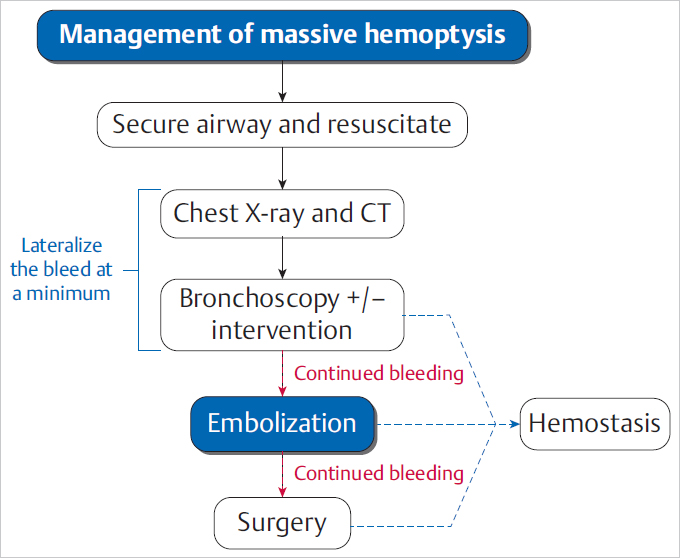
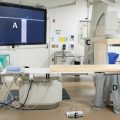
5 Emergency IR Management of bleeds accounts for the vast majority of IR emergencies. Bleeding can occur essentially anywhere in the body, including the GI tract, solid abdominal organs, retroperitoneum, and superficial soft tissues. Embolization is a minimally invasive and versatile tool for addressing hemorrhage in emergencies. Hemoptysis ranges in severity from blood-tinged sputum to gross hemorrhage. Massive hemoptysis can be life threatening, usually as a result of asphyxia rather than hemorrhagic shock. Massive hemoptysis is defined as greater than 500 mL of blood coughed up in a 24-hour span or a rate of 100 mL/h. Most cases of hemoptysis originate from the bronchial arteries, though it can also, rarely, originate from the lower-pressure pulmonary arterial system (▸Fig. 5.1). Fig. 5.1 Posterior view of the thoracic aorta showing the most common bronchial artery anatomy, with a single right bronchial artery arising from the intercostobronchial trunk, and two left bronchial arteries arising directly from the aorta. Origins are typically between T3 and T5. (Source: 6 Bronchial Arteries (Rami Bronchiales). In: Wacker F, Lippert H, Pabst R, eds. Arterial Variations in Humans: Key Reference for Radiologists and Surgeons. 1st Edition. Thieme; 2017.) When a patient presents with suspected hemoptysis, one of the first steps is deciding whether it is true hemoptysis or if the source of bleeding originates from the oropharynx or GI tract (▸Fig. 5.2). Physical examination alone does not usually allow differentiation between hemoptysis and hematemesis, but bleeding from the oropharynx or nasal passages may be more obvious. Consideration of patient risk factors including history of smoking, cystic fibrosis (CF), or vasculitis can help sway your suspicion toward hemoptysis when the diagnosis is uncertain. If the bleeding is thought to be hemoptysis, the underlying cause and laterality are then determined. The most common causes of hemoptysis include infections, lung malignancies, and diseases associated with bronchiectasis such as CF. An initial chest X-ray may help in localizing the bleed to either the right or left lung, but the majority of these patients will undergo a chest CT as well. Neoplasms, cavitary lesions, pulmonary infarcts, and bronchiectasis may be evident on CT. Bronchoscopy is usually performed following patient stabilization. The minimum goal is to establish which lung (and possibly which lobe) is bleeding. If the laterality is determined, the patient should be positioned lying with the affected lung down. This prevents blood from spilling into the contralateral lung. Although not essential, CTA can help confirm the source of the bleed, and should be considered on a case-by-case basis, either before or after bronchoscopy. In addition to active bleeding, CTA can identify the number and location of the bronchial arteries and any variant arterial anatomy, which is useful information to have when evaluating for an intervention. Prior to any diagnostics or intervention, patients with massive hemoptysis require fluid resuscitation. Intubation is often required to protect the airway. As many cases of hemorrhage occur peripherally in the lung, bronchoscopy is primarily a diagnostic procedure, though some bronchoscopic treatments exist. Interventions include laser coagulation, electrocautery, and local hemostatic agents (e.g., epinephrine, thrombin, etc.). In the case of unilateral hemorrhage, a bronchial blocker is sometimes used by the bronchoscopist as a temporizing measure. This involves inflating a balloon within a bronchus of the bleeding lung to prevent spillage of blood into the unaffected airways. Conventional angiography with embolization has become first-line therapy for severe cases of hemoptysis, since bronchoscopy has limited therapeutic potential, and emergent intrathoracic surgery is associated with high perioperative mortality (Procedure Box 5.1, Procedure Box 5.2). Surgery (wedge resection/lobectomy/pneumonectomy) is reserved for patients who fail more conservative options. Patients treated with bronchial artery embolization are monitored closely in a critical care setting. Extubation should be considered only when the risk of rebleeding is low. Trending hemoglobin is not that useful, as small-volume hemorrhages can still cause asphyxia without a significant effect on the hematocrit values. In cases where an underlying diagnosis remains elusive, bronchoscopy is often repeated after embolization to better evaluate the airways. Rebleeding is not an uncommon problem, particularly in CF patients. A bronchial angiogram involves catheterizing the bronchial artery origins; these are usually found in the midthoracic aorta, near the mainstem bronchi. On all bronchial arteriograms, careful and deliberate attention should be paid to identify any medullary arteries communicating with the anterior spinal artery, particularly the artery of Adamkiewicz (great anterior radiculomedullary artery), which typically arises from an intercostal artery between T9 and T12 and provides the largest supply to the anterior spinal cord. Medullary arteries demonstrate a characteristic pattern, with an upward course followed by a hairpin loop prior to entering the anterior spinal artery. Inadvertent embolization of a spinal artery may result in severe neurological deficits, including para-plegia. Bronchial arteries with a large spinal branch can be treated, but a microcatheter needs to be positioned beyond this branch, with meticulous attention to avoid reflux. In patients with necrotizing pneumonia or bronchiectasis due to CF, collateral arteries may grow toward the site of insult to perfuse and repair. These novel collaterals may arise from multiple systemic arteries, including the internal mammary, thyrocervical, intercostal, lateral thoracic, and phrenic arteries. These “parasitized arteries” are prone to hemorrhage. Evaluation of a preangiogram CTA can be useful to identify parasitized arteries which may require angiographic interrogation and possible embolization. Angiographic findings such as abnormally tortuous or hypertrophied vessels, hypervascular lung parenchyma, pseudoaneurysms, or contrast extravasation into airways all indicate sources of hemoptysis. Particles used for embolization are typically 500 to 700 µm in size (larger than most spinal arteries) and are considered front-line embolic agents for hemoptysis. Particles embolize distally within the targeted arterial tree, wedging in the small arteries associated with the underlying tumor, infection, etc. Use of particles allows for repeat intervention should hemoptysis recur. Coils are less desirable since reintervention in the same vessel beyond a deployed coil may be difficult or impossible. The indications, technique, and potential complications of pulmonary artery embolization vary considerably compared to those of bronchial artery embolization. In pulmonary artery embolization, the common femoral vein is accessed and the pulmonary outflow tract is selected with a special curved pigtail catheter, such as a Van Aman or Grollman. Once the right or left pulmonary artery is selected, pulmonary angiography is performed. Most frequently, the target for pulmonary artery embolization is a pulmonary arteriovenous malformations (AVM). Pulmonary artery pseudoaneurysms may also be implicated, particularly in the setting of previous Swan–Ganz catheter placement or mycobacterial infection. In contrast to bronchial artery embolization, coils and vascular plugs are the preferred embolic agents for pulmonary artery embolizations. Pulmonary AVMs create right to left shunts by bypassing the intervening pulmonary alveolar capillary network. If particles or glue are deployed into an AVM, these agents are small enough to pass directly into the pulmonary venous outflow due to the lack of intervening capillaries. Nontarget embolization in the systemic circulation can cause devastating complications such as stroke, visceral infarction, and limb ischemia. Embolic agents should be carefully selected for embolization of AVMs in order to prevent migration through the malformation into the pulmonary veins. Surgery plays an adjunctive role for certain patients after embolization. If the cause of the hemoptysis is due to a mass lesion or mycetoma, wedge resection and lobectomy are viable options, provided the patient is a surgical candidate. When recurrent hemoptysis is due to diffuse pulmonary disease (CF, sarcoidosis, vasculitis), lung transplant remains an option of last resort. Bleeding from the GI tract proximal to the ligament of Treitz is considered upper GI bleeding (UGIB), whereas bleeding distal to it is considered lower GI bleeding (LGIB). Although UGIB can result in serious hospitalizations, they are less frequently encountered by the IR. Upper endoscopy (EGD) is usually successful in managing UGIB. Endovascular intervention is, however, an important tool for refractory UGIB. Both arterial and venous forms of UGIB can occur; the latter typically a consequence of gastroesophageal varices (discussed separately in Chapter 6). Peptic ulcers are the most common cause of UGIB. Bleeding occurs when ulcers penetrate beyond the mucosa into the submucosa, causing inflammation, necrosis, and eventual rupture of an artery. Helicobacter pylori infection, NSAID use, and hemorrhagic gastritis are also common culprits. Other causes of UGIB are listed in ▸Table 5.1. Table 5.1 Arterial causes of upper GI bleeding
5.1 Massive Hemoptysis
Approach to Massive Hemoptysis
Management of Hemoptysis
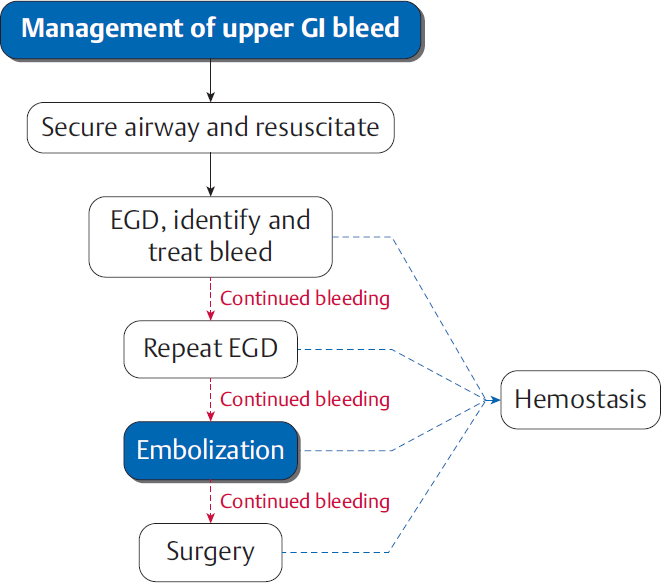
5.2 Upper GI Bleeding
• Peptic ulcers (gastric or duodenal) • Mallory–Weiss tear • Gastritis/duodenitis/esophagitis • Dieulafoy’s lesion | • Gastric cancer • Angiodysplasia • Hemobilia (iatrogenic) • Aortoduodenal fistula |
Work-up of an UGIB
Hematemesis and/or melena are the most common presenting symptoms of UGIB. Hematochezia is more likely to come from a lower GI bleed, but can be seen due to a bleed from above with rapid transit through the GI tract. Patients with these brisk bleeds are more likely to be hemodynamically unstable and require emergent intervention. If the presence of UGIB is uncertain, gastric fluid can be lavaged via nasogastric (NG) tube and tested on a stool guaiac strip, although this will miss bleeding that occurs within the proximal duodenum.
Certain clues can help direct the clinician toward a cause of the bleed. Peptic ulcers cause epigastric pain and early satiety, and may present with a history of NSAID use or prior ulcer disease. Mallory–Weiss tears should be suspected when nonbloody emesis precedes hematemesis in an alcoholic patient. Hemorrhagic gastritis produces coffee-ground hematemesis, usually in an ICU patient or someone with a history of chronic steroid use.
Hypotension and hemodynamic shock are imminent threats to life in cases of GI bleeding. Medical management to support blood pressure should always take priority over further diagnostic work-up (▸Fig. 5.3).
If endoscopy fails to reveal a source of the bleeding, CTA can be performed for localization. CTA offers high-resolution, cross-sectional imaging of the GI tract and its arterial supply. It also has a high sensitivity, detecting bleeds as slow as 0.3 mL/min (compared to 0.5 mL/min by conventional angiography). Hyperdense contrast material that layers within the bowel lumen on arterial phase and increases on delayed phase is indicative of active bleeding. Radionuclide scintigraphy, also known as a tagged red blood cell (RBC) scan, is rarely used in cases of UGIB. Both CTA and scintigraphy are less commonly used for diagnosis of a suspected UGIB, since endoscopy is usually effective at identifying a source.
Management of UGIBs
The airway should always be secured and hemodynamics controlled before taking a patient to endoscopy or angiography for intervention.
In preparation for endoscopy, the patient should be made NPO and placed on an intravenous proton pump inhibitor (PPI), such as pantoprazole. PPIs promote clot formation within the GI tract and reduce rates of rebleeding.
Endoscopic tools may achieve hemostasis by physical, chemical, or thermal means. Physical occlusion of a vessel is performed using metallic clips or rubber bands. Chemical injection of vasoconstrictors, such as epinephrine, are typically more effective when used in conjunction with other therapies. Thermocoagulation works similar to electrocautery.
Patients with UGIB present to IR either because attempts at endoscopic hemostasis have failed, or because the patient is too unstable for evaluation by endoscopy. Cases of suspected or known hemobilia will often proceed directly to endovascular intervention as well. Many IRs prefer for the patient to have a CTA prior to the procedure. It doesn’t always identify the location of the bleed, but when it does, the embolization procedure has a much greater chance of being successful. Embolization of UGIBs generally have a high success rate, and can be repeated if the initial study is negative or in cases of rebleeding. (Procedure Box 5.3).
Surgery is reserved for those who have failed both endoscopic and endovascular management. Roughly a quarter of UGIB cases that are treated by IR will go on to eventually require surgery. The operative procedure depends on the cause of UGIB, and ranges from ligation of bleeding arteries to resection of a portion of the stomach or duodenum. Patients with uncontrolled hemobilia may undergo liver resection in extreme cases.
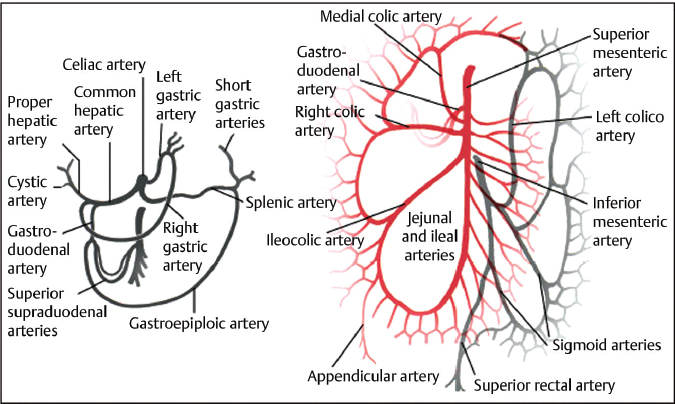
After obtaining femoral or radial artery access, selective catheterization of the celiac artery is generally the first step. The key branches to the stomach include the left and right gastric and right gastroepiploic arteries (▸Fig. 5.4). Additionally, a key branch supplying the duodenum is the gastroduodenal artery (GDA), which usually arises from the common hepatic artery and gives off the superior pancreaticoduodenal artery (PDA).
After evaluation of the celiac axis, the superior mesenteric artery (SMA) is then investigated. Its key branch to the proximal small bowel is the inferior PDA. The superior and inferior PDAs form the pancreaticoduodenal arcade, which allows for collateral flow between the celiac and SMA. This is a useful redundancy in case of an occlusion, but can cause problems with recurrent bleeding after embolization. Bleeding that continues following embolization as a result of one of these collaterals is often referred to as a “back door” bleed. Understanding the vascular anatomy in this region is essential in planning an embolization.
The direct angiographic sign of active UGIB is contrast extravasation and pooling within the stomach or proximal bowel (▸Fig. 5.5). Indirect signs suggesting a vascular abnormality include pseudoaneurysm, vessel spasm, or early filling of the venous outflow. A contrast “blush” or area of unexpected hypervascularity could represent an inflammatory process or neoplasm as the underlying cause.
Once a bleed is identified, the goal is to selectively decrease perfusion to the site of arterial injury via embolization, allowing for thrombosis or endogenous vessel repair to occur. Coils, Gelfoam, glue, and particles can all be used to treat UGIB. Coils are used most commonly because they are inexpensive and can be deployed with precision. Bleeds arising from a well-developed collateral pathway require coil embolization proximal and distal to the injury. Particles may be used if the bleed originates from an inaccessible distal branch, or if a bleeding tumor needs to be devascularized. In general, permanent embolic agents can be used with minimal concern for gut ischemia when treating UGIBs due to the excellent collateral blood supply. Gelfoam, as a more temporary agent, is often used in combination with coils, but rarely in isolation. It degrades over time, which allows vessels to recanalize within weeks. The risk of bowel ischemia and nontarget embolization are higher when using particles and glue, often when patients have diminished collateral blood flow as a result of prior surgery.
In some cases, empiric embolization of a gastric artery or the GDA may be considered if the angiogram is negative, but a clear source of bleeding was localized to the territories supplied by those arteries on endoscopy.
5.3 Lower GI Bleeding
As with UGIBs, LGIBs can come from both arterial and venous sources. Venous bleeding is usually associated with mesenteric variceal hemorrhage or hemorrhoids. This distinction is important whenever fielding a LGIB consult, as the interventions IR offers for venous hemorrhage is decidedly different often more limited. This section will focus on arterial causes of LGIB.
LGIB can be subdivided into those arising from small intestine and those arising from colon or rectum. Bleeding from the small intestine is often more complex to manage, given the wider variety of contributing pathologies and the anatomy that is often inaccessible by endoscopy.
AVMs, malignancies, diverticuli, ulcers, trauma, and forms of enteritis such as Crohn’s disease can all be responsible for a small bowel LGIB (▸Fig. 5.6). Bleeding from the large intestine occurs most commonly due to diverticuli and angiodysplasia (tiny, usually age-related AVMs). Less common causes include tumors, colitis, and recent interventions (e.g., polypectomy) (▸Table 5.2).
Fig. 5.4 Diagram of the arterial supply to the visceral organs and relationship to adjacent structures. Key branches include the left gastric, right gastric, left gastroepiploic, and right gastroepiploic arteries.
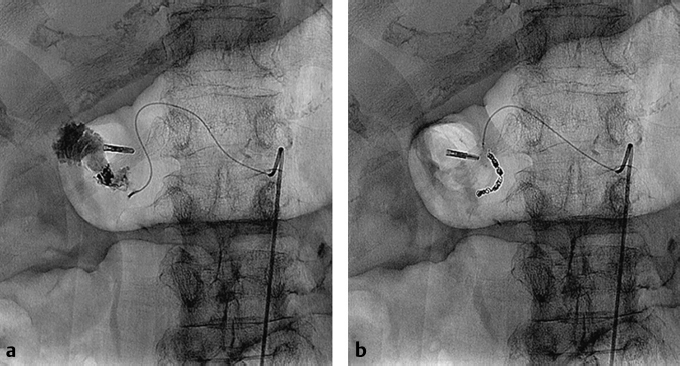
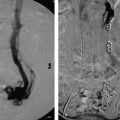
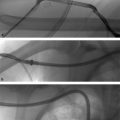
Fig. 5.5 (a) A catheter is parked in the celiac axis with a microcatheter in the gastroduodenal artery (GDA). An angiogram demonstrates active contrast extravasation from the GDA. (b) Coil embolization was performed.
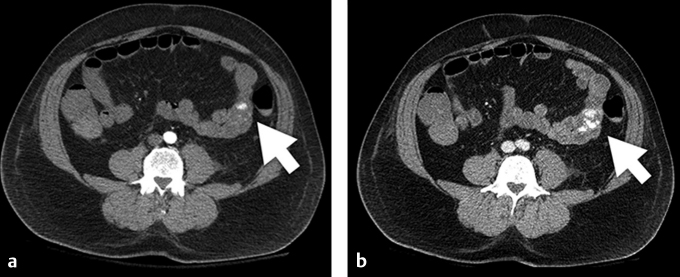
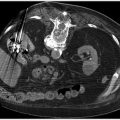
Fig. 5.6 (a) Multiphasic CT demonstrates arterial phase contrast pooling within the lumen of the midjejunum. (b) Additional contrast is seen pooling on venous phase imaging, indicating active extravasation. The bleeding was due to a jejunal arteriovenous malformation. (These images are provided courtesy of Matthew Evan Krosin, MD, University of Pittsburgh Medical Center.)
Table 5.2 Arterial causes of lower GI bleeding
Small intestine (jejunum and ileum) • Enteritis • Ulcer • Tumor • Arteriovenous malformation (AVM) • Trauma • Diverticulum (less common) • Aortoenteric fistula (rare) | Large intestine (colon and rectum) • Diverticuli • Angiodysplasia • Tumor • Colitis • Postprocedural (polypectomy, biopsy) |
Work-up of a Lower GI Bleeding
Hematochezia is the typical presentation of a LGIB, though it can also be seen with a brisk UGIB. Melena is uncommon in LGIB, as bleeding distal to the ligament of Treitz does not mix with the gastric and pancreatic juices that cause oxidation and darkening of the blood. However, melena can be seen with a proximal LGIB or when there is exceptionally slow transit time.
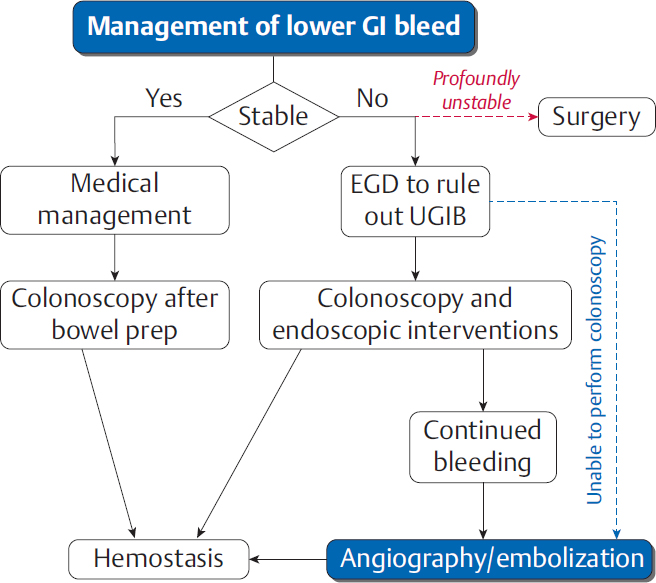
As with UGIBs, resuscitation should be carried out simultaneously with the initial work-up for a LGIB. A colonoscopy is almost always indicated, but the timing depends on the urgency of the case (▸Fig. 5.7). Patients who are stable after resuscitation are generally admitted and given a bowel prep prior to colonoscopy. Hemodynamic instability and ongoing bleeding may require only an abbreviated prep, and many endoscopists are hesitant to perform the procedure without a prep. LGIB patients are generally more stable than UGIB patients; severe hematochezia and instability should arouse suspicion for an UGIB. These patients get an EGD or NG tube aspiration to rule out a bleed from above prior to colonoscopy.
Colonoscopy does not always successfully identify a source of hemorrhage due to technical difficulty and/or the presence of stool in a suboptimally prepped colon. When endoscopy is unsuccessful and the patient is hemodynamically stable, a CTA may be done to help localize the bleed. CTA can determine bleeding location, underlying pathology, and vascular anatomy.