CHAPTER 49 Emerging Concepts of Cerebrospinal Fluid Physiology and Communicating Hydrocephalus
New data are modifying our classic concepts of cerebrospinal fluid (CSF) production, flow, and reabsorption.1,2 Consequently, they are calling into question our present understanding of the cause and nature of hydrocephalus.3–5 Classically, hydrocephalus has been considered to result from an imbalance in the formation, flow, or absorption of CSF with abnormally increased CSF volume and/or pressure. Increasingly, hydrocephalus is now believed to result from disordered pressure/volume relationships (compliance) within the intracranial-intraspinal compartments.3–5 The information presented here builds on that in Chapter 48 to present some emerging concepts of CSF physiology and hydrocephalus. In some cases, the data appear conflicting. Nonetheless, it is hoped that a broader understanding of normal and deranged CSF physiology may lead to improved diagnosis and treatment of hydrocephalus in the future.
The following definitions are provided for a better understanding of the concepts discussed:
Cerebrospinal fluid is the fluid within the ventricles and cisterns of the intracranial and intraspinal spaces.
Interstitial fluid (ISF) is the extracellular fluid of the brain derived from the blood, the metabolic activity of the brain, and recycled CSF.
Compliance (C) is the ratio of the volume change (ΔV) to the pressure change (ΔP), given as C = ΔV/ ΔP. In a highly compliant system, a large increase in volume will cause only a small change in pressure. In a rigid, less compliant system, a small increase in volume may lead to a large increase in pressure.
Hydrocephalus is a condition in which there is an increase in the total volume of CSF and in which that increased volume of CSF is associated with elevated intracranial pressure, presently, intermittently, or in the past (Fig. 49-1).
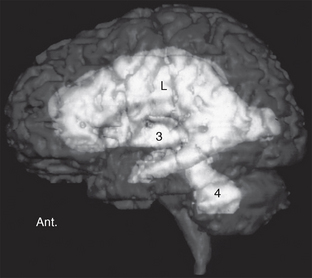
FIGURE 49-1 Left lateral view of a volumetric MRI study. The disproportionate enlargement of the lateral (L), third (3), and fourth (4) ventricles indicates increased CSF volume. Increased CSF volume under pressure is hydrocephalus. Ant., anterior.
PHYSIOLOGY
CSF Volume
Classically, the total volumes of CSF have been given as 40 to 60 mL in infants, 60 to 100 mL in children, and about 150 mL in adults.6–8 The volume of the adult ventricles has been considered to be 25 to 30 mL. Recent work now suggests that the total CSF volume is much higher: perhaps 150 mL in the intracranial subarachnoid space, 100 to 120 mL in the spinal subarachnoid space, plus 100 to 300 mL of interstitial fluid within the central nervous system.9–12
Interstitial Fluid Production
Interstitial fluid is most likely produced by active transport across the capillary endothelium and as a by-product of the metabolic activity of the brain (Figs. 49-2 and 49-3).12–14 A portion of the interstitial fluid may also be recycled CSF that reenters the brain via the arterial perivascular spaces along the ventral surface of the brain.12
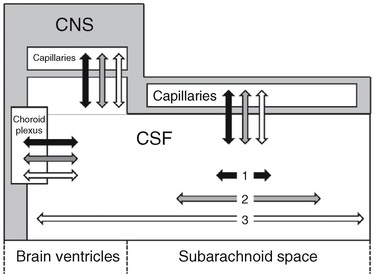
FIGURE 49-2 Communication among the intracranial fluid compartments. Schematic representation. Water (two-headed black arrows) exchanges freely between the CSF and the capillaries, has a short half-life in the CSF, and has very limited distribution along the CSF spaces (short horizontal black arrow 1). Substances with very restricted transit from CSF to capillaries (such as 3H-inulin; two-headed white arrows) have long half-lives in the CSF and unlimited bidirectional distribution along the CSF spaces (long horizontal white arrow 3). Substances with intermediate rates of passage from the CSF to the capillaries, such as organic acids (two-headed gray arrows) have limited half-lives in the CSF and more limited distribution along the CSF spaces (intermediate horizontal gray arrow 2). The spread along the CSF in both directions is presumably due to CSF pulsations.
(From Bulat M, Klarica M. Recent insights into a new hemodynamics of the cerebrospinal fluid. Brain Res Rev 2011; 65:99-112.)
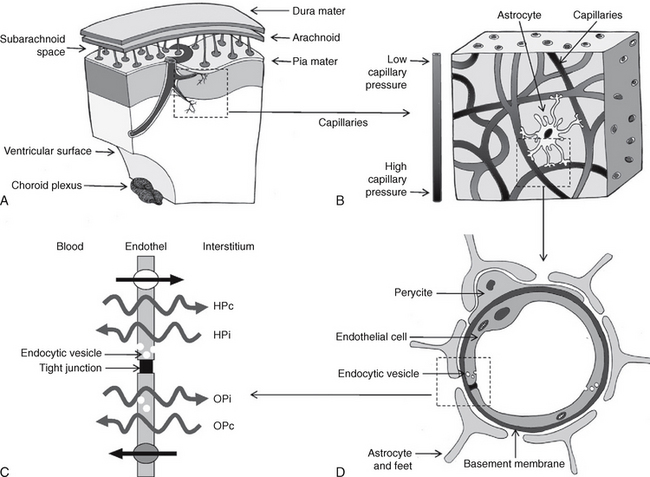
FIGURE 49-3 Proposed mechanism of CSF hydrodynamics. Schematic representation. A, The vessels penetrate deep into the brain. B, As they pass through the brain, the proximal capillaries have a high perfusion pressure, while the distal capillaries have low perfusion pressure. C and D, At the distal capillary, the endothelial cell is surrounded by pericytes, the foot processes of astrocytes, and the basement membrane, which contribute to the blood-brain barrier. The endothelial cell membrane of the capillary wall is variably permeable to different substances. The net passage of water depends on the gradients between (1) the hydrostatic capillary pressure (HPc) versus the hydrostatic pressure of the interstitium (HPi) and (2) the osmotic capillary pressure (OPc) versus the osmotic pressure of the interstitium (OPi). Large, more hydrophilic molecules may pass from the blood into the interstitial fluid (straight arrow at top). Transport systems also permit the return of molecules from the interstitium to the blood (straight arrow at bottom). Molecules of large molecular weight traverse the endothelial cell wall via endocytic vesicles (small white circles).
(From Oreškovic D, Klarica M. The formation of cerebrospinal fluid: nearly a hundred years of interpretations and misinterpretations. Brain Res Rev 2010; 64:241-262.)
CSF Production
Classically, 60% to 90% of CSF was thought to be produced within the ventricular system by the choroid plexus and 10% to 40% within the brain by active transport across the capillary endothelium into the brain.7,8 Recent work now suggests that fluid is produced everywhere in the central nervous system (see Figs. 49-2 and 49-3).1,2 Much of that fluid forms first as interstitial fluid, which then rapidly exchanges and mixes with the CSF across the pia, ependyma, and perivascular spaces.1,2,12 Chemically, the interstitial fluid and CSF have very similar compositions and are nearly indistinguishable from each other.13 The volume of CSF produced by the spinal cord is considered to be negligible.
Quantitatively, CSF production has been estimated at 0.305 ± 0.145 mL/min or about 600 mL/day.15 This volume markedly exceeds the ventricular CSF volume of 25 to 30 mL but is small when compared with the cerebral blood flow of 750 mL/min. The turnover time for CSF is 5 to 7 hours, so the total CSF volume is renewed about 4 times a day.11 The rate of fluid secretion by the choroid plexus depends, in part, on the perfusion pressure of the blood. With rising intracranial pressure, CSF production decreases.2
CSF Reabsorption
Classically, most CSF was believed to be reabsorbed within the head through the arachnoid granulations situated along the dural venous sinuses (85% to 90%). The rest was believed to be absorbed within the spine along the root sheaths of the spinal nerves (10% to 15%).16,17
Head
The arachnoid villi/granulations have been thought to act as mechanical valves to regulate passage of CSF into the dural venous sinuses.18 Di Chiro demonstrated that radioisotope injected into the lumbar CSF passed superiorly within the CSF and accumulated over the cerebral convexities in relation to the arachnoid granulations.19 This was taken to be support for the role of the arachnoid granulations in CSF reabsorption.19 To date, however, no experimental evidence actually proves that fluid is transported across the arachnoid villi in normal individuals. Indeed, in children, arachnoid granulations do not develop until after the fontanelles close, so, in children, CSF reabsorption must be accomplished by a different mechanism.20
Spine
It is known that radiolabeled albumin injected into the lumbar subarachnoid space becomes detectable in the blood stream just minutes after injection, well before it could ascend through the spinal canal into the head.bib14–311 Bozanovic-Sosic and colleagues tried to estimate the percent clearance of CSF from the intracranial versus the intraspinal compartments by placing an extradural ligature around the thecal sac between C1 and C2 and instilling into the CSF human serum albumin radiolabeled with two different isotopes, one isotope for each compartment. They found that the spinal compartment accounted for approximately 25% of the total clearance of the albumin.16
Alternate Pathways
Alternate pathways for CSF reabsorption have been documented in animals and humans.20–27 Physiologic evidence suggests that these alternate pathways are a significant route, perhaps the dominant route, for CSF reabsorption at normal CSF pressures.28 With elevated pressure, absorption may take place via the arachnoid granulations. At elevated pressures, CSF may also pass into the dura through “clefts” that convey the CSF to the venous sinuses.16,29,30
Lymphatic Drainage
There are no conventional lymphatic channels within the brain. Nonetheless, there is a substantial flow of interstitial fluid and CSF from the brain to the cervical lymph nodes. In animals such as rodents and sheep, up to 50% of CSF drains from the subarachnoid space to the cervical and lumbar lymph nodes along cranial and spinal nerve roots (Fig. 49-4).24 The percent in humans is not yet established.
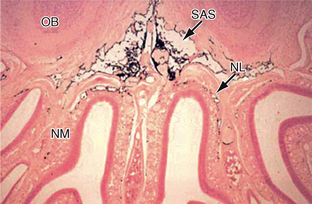
FIGURE 49-4 Drainage of CSF into the nasal lymphatics (NL) of the rat. India ink (black) placed into the subarachnoid space (SAS) drains under the olfactory bulbs (OB) and through the cribriform plate into the lymphatics of the nasal submucosa (NM).
(From Weller RO, Djuanda E, Yow H-Y, Carare RO. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol 2009; 117:1-14.)
Weller has shown that the interstitial fluid and the CSF drain to the cervical lymph nodes by two different paths.23,24
Drainage of Interstitial Fluid (Path 1)
Interstitial fluid and solutes appear to drain from the brain along the 100 to 150 nm wide basement membranes in the capillary walls and then continue onward along the basement membranes between the smooth muscle cells in the tunica media of the arteries (Fig. 49-5).23,24 The interstitial fluid and solutes then enter the adventitia around the leptomeningeal arteries and continue through the base of the skull along the carotid artery (and probably the vertebral artery) to the cervical lymph nodes.23 A layer of pia-arachnoid separates the adventitia of the leptomeningeal arteries from the CSF in the subarachnoid space.23
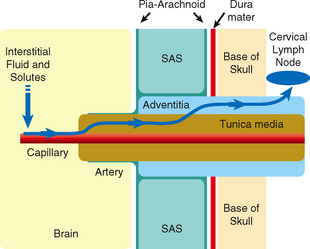
FIGURE 49-5 Diagram of the perivascular route for drainage of interstitial fluid from the brain into the lymphatic system. From left to right, interstitial fluid and solutes drain from the brain parenchyma into the basement membranes of capillaries. From there they pass along the basement membranes between smooth muscles of the tunica media of arteries and continue into the adventitia of the leptomeningeal arteries. They then continue through the skull base along the carotid (and probably the vertebral) arteries to the cervical lymph nodes. A layer of pia-arachnoid separates the adventitia of the leptomeningeal arteries from the CSF in the subarachnoid space (SAS).
(From Weller RO, Djuanda E, Yow H-Y, Carare RO. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol 2009; 117:1-14.)
The motive force for this “lymphatic” flow appears to be the pulse wave traveling along the arteries.23 Perivascular lymphatic drainage is seen only in living animals and ceases immediately after cardiac arrest.24 It likely proceeds as follows: with each pulsation, an arterial pressure wave passes antegrade along the vessel. After each pulse wave there is a contrary (or reflection) wave traveling in the reverse direction—retrograde. This contrary wave may drive the perivascular lymphatic drainage out of the brain. A valve-like action to prevent backflow may be provided by the change in the configuration of the basement membrane from its sheet-like, stretched, distended position in systole to its folded recoil position in diastole.23 Normal innervation of the vessels is also needed for perivascular “lymphatic” flow. In rabbits, cholinergic deafferentation of these vessels appears to reduce perivascular drainage.23
Drainage of CSF (Path 2)
The CSF appears to drain to the lymphatic system through a direct connection between the subarachnoid space and the lymphatic channels that pass down through the cribriform plate next to the olfactory nerves to reach the nasal submucosa (Fig. 49-6).23,24 Multiple lymphatic ducts form a collar around the emerging nerve roots. This collar facilitates direct connection between the CSF and lymph in the olfactory submucosa.26 These two routes for drainage of interstial fluid and CSF may become intertwined when there is impaired drainage through either one of them.23,24
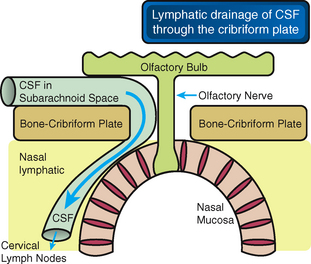
FIGURE 49-6 CSF drains into the nasal lymphatics. The cerebrospinal fluid (CSF) within the subarachnoid space drains into distinct lymphatics that pass under the olfactory bulbs and through the ostia in the cribriform plate to reach the lymphatics in the nasal submucosa.
(From Weller RO, Djuanda E, Yow H-Y, Carare RO. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol 2009; 117:1-14.)
Parenchymal Veins
CSF reabsorption may also take place through the parenchymal veins. It is now accepted that there is full physiologic continuity and exchange between the interstitial fluid and the CSF at the walls of the brain capillaries (see Fig. 49-2).12–14 The surface area of the cerebral capillaries has been estimated at 240 cm2 per gram of tissue.31 For a human brain of 900 to 1500 grams (wet weight, unfixed), this predicts a very large surface area of vessels to absorb interstitial fluid (perhaps 200,000 to 350,000 cm2).31 The surface area of the arachnoid villi and perineural sheaths is not more than 10 cm2.2 The parenchymal capillaries are known to actively absorb the macromolecules and plasma proteins present in the CSF and regulate fluid homeostasis at a slightly positive intracranial pressure. Therefore, it is likely that CSF may also be reabsorbed across the walls of the parenchymal veins (see Fig. 49-3). Interestingly, aquaporin-4 is upregulated in hydrocephalus, further suggesting the CSF is absorbed into the blood from the edematous periventricular white matter.23
3H-Water
When 3H-water is infused slowly into the lateral ventricle of a cat, it is reabsorbed rapidly across the ventricular wall into the adjacent capillaries, the vein of Galen and torcular, which then manifest a very high concentration of 3H-water (Fig. 49-7).13,14 The reabsorption within the ventricles is so high that little 3H-water passes out of the lateral ventricles to reach the cisterna magna.13,14 That is, there is little to no bulk flow of water from the ventricles into the basal cisterns.13,14
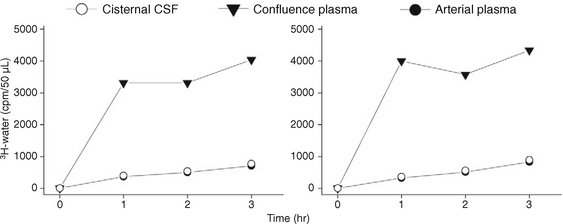
FIGURE 49-7 Infusion of tritiated water in saline into the lateral ventricle of a cat at high concentration (0.125 mCi/mL) disclosed rapid reabsorption of the tracer into the venous system (high concentration in the plasma at the dural venous confluence) with very low levels of tracer in the cisternal CSF (equal to the levels in the arterial plasma).
(From Bulat M, Lupret V, Oreškovic D, Klarica M. Transventricular and transpial absorption of cerebrospinal fluid into cerebral microvessels. Coll Antropol 2008; 32[Suppl 1]:43-50.)
3H-Inulin
Conversely, when the macromolecule 3H-inulin is instilled into the ventricular CSF, it enters the bloodstream very slowly. As a consequence it persists within the ventricles and, with time, is carried by systolic-diastolic pulsations, bidirectionally, along the ventricular system to the basal cisterns (Fig. 49-8).13,31 The concept that volumes of CSF pass through the ventricles to be reabsorbed distally within the basal and convexity cisterns may be an artifact of the macromolecules used to study CSF volume hydrodynamics (flow). The data derived from macromolecule studies may misrepresent the physiology of the 99% of CSF that is low molecular weight water.13,14
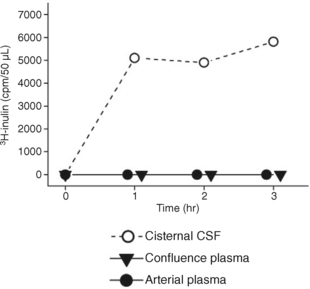
FIGURE 49-8 Infusion of tritiated inulin in saline into the lateral ventricle of a cat at high concentration (0.018 mCi/mL) disclosed very slow reabsorption of the tracer into the venous system (concentration of tracer in the confluence plasma equal to the concentration of tracer in the arterial plasma). High levels of tracer persisted within the CSF that passed through the ventricular system into the cisternal CSF.
(From Klarica M, Oreškovic D, Božic B, et al: New experimental model of acute aqueductal blockage in cats: effects on cerebrospinal fluid pressure and the size of brain ventricles. Neuroscience 2009; 158:1397-1405.)
Physical Activity
The rate of absorption of macromolecules within the CSF also depends, in part, on the physical activity of the person or experimental animal. Mixing of tritiated inulin within the CSF and its bidirectional distribution throughout the CSF volume is greatly increased by respiratory and body movements.31 Similarly, in patients injected with the radionuclide tracer technetium-99m-labeled diethylenetriaminepentaacetic acid (99mTc-DTPA), the rate of absorption of the tracer is higher in active than resting individuals. In one experimental study of radionuclide tracer injected into the lumbar spinal canal, the activity decreased 20% (±13%) in the first hour, higher in active than resting individuals, with no correlation between radionuclide reduction, intraspinal movement of the tracer, or the rate of CSF production.17
Low Pressure Versus High Pressure
The sites and proportions of CSF absorption may vary with the intracranial pressure. For example, when radioiodinated serum albumin (RISA) is infused into the CSF of cats and rabbits under normal pressure, it stays mainly in place. When the RISA solution is infused under increased CSF pressure, however, the RISA shows a greatly increased concentration in the optic nerves, olfactory bulbs, episcleral tissue, and deep cervical lymph nodes.31 When a solution of horseradish peroxidase (HRP) is infused into the CSF under normal CSF pressure, it remains within the CSF. When the solution is infused under high pressure, the high pressure damages the layers of arachnoid connected by tight junctions adjacent to the dura and the HRP then enters the highly vascularized dura. Furthermore, when HRP is infused under high pressure (1) the endothelial cells of the arachnoid villi develop vesicles and transcellular channels and (2) the resistance to fluid infusion decreases greatly, indicating nonphysiologic increase in fluid absorption.31 Nagra and associates concluded that “the available data suggest that CSF transport can occur into the cranial venous system but only at high intracranial pressures.”32–34
Compliance and Pressure/Volume Relationships
Compliance (C) is the ratio of a volume change (ΔV) to the pressure change (ΔP) that is induced by that incremental change in volume. Compliance is given as C = ΔV/ΔP.1 In a highly compliant system, a large increase in volume will cause only a small change in pressure. That is, the compliant brain can accommodate a substantial increase in ventricular volume with little increase in intracranial pressure. In a less compliant system, the brain is more rigid, so even small increases in volume cause large increases in intracranial pressure.
This pressure/volume relationship is exponential (Fig. 49-9).1 The change in pressure caused by any increment in volume increases exponentially as the mean pressure rises. Put differently, as the mean pressure rises, the system becomes more rigid and more sensitive to slight variations in volume.1 For the brain, the overall compliance is composed of the actual compliance of the brain tissue (small), the arterial compliance, the venous compliance, and the compliance of the spinal thecal sac.
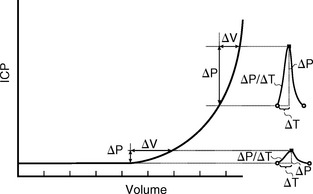
FIGURE 49-9 The pressure/volume curve is exponential. At normal intracranial pressures (ICP), the systolic pulse of pressure and volume that enters the head produces only a small increase in ICP (about 1 mm Hg). As ICP rises, the intracranial compliance decreases, causing a marked increase in the pulse pressure, even if there is no change in the arterial pulse of pressure and volume that enters the head.
(Adapted from Marmarou A, Shulman K, LaMorgese J: Compartmental analysis of compliance and outflow resistance of the cerebrospinal fluid system. J Neurosurg 1975; 43:523-534.)
Hydrodynamics of CSF
It remains uncertain whether the passage of CSF through the ventricular system results from (1) bulk flow driven down a pressure gradient from the ventricle to the subarachnoid space, (2) flow swept onward by the coordinated beating of the ciliated ependyma of the ventricular walls, and/or (3) rhythmic flow generated by the cycles of cardiac pulsation.35,36
Bulk Flow of CSF
Dandy35 showed that plugging the aqueduct in dogs caused dilation of the ventricles proximal to the obstruction and took this as evidence for bulk directional flow of CSF. Bulat and colleagues question the very concept of bulk flow of CSF through the ventricular system.13,14
Pulsatile Forces in the CSF
Increasingly, it is believed that cyclic pressure/volume waves generated by the cardiac contractions pass into the intracranial space, rhythmically distend the intracranial arteries and capillaries, expand the brain, and thereby control CSF flow (Fig. 49-10).37–40 Other pulsatile variations such as respiratory and vasomotor-induced oscillations do affect the pressure and flow over time but have a small effect compared with the cardiac pulsations.1
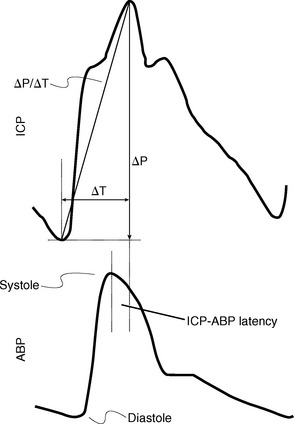
FIGURE 49-10 Relationship of the intracranial pulse wave engendered by the arterial pulse. Comparison of the two waves shows the temporal difference (ΔT) in the arterial versus ICP waves, which can be given as the latency (phase offset) of the ICP versus the arterial wave (ICP-ABP latency).
(From Wagshul ME, Eide PK, Madsen JR. The pulsating brain: a review of experimental and clinical studies of intracranial pulsatility. Fluids Barriers CNS 2011; 8:5.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree