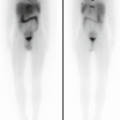
Fig. 7.1
Thyroid ectopy
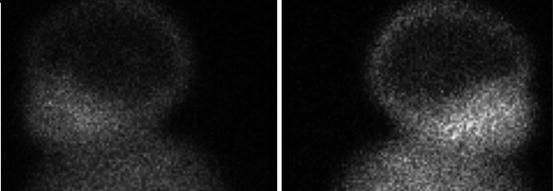
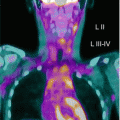
Fig. 7.2
Thyroid agenesis
On the other side, 123I can be of more difficult availability and of higher costs. It is trapped and organified by the thyroid gland [5]. The sensitivity of TS when using 123I is higher than with 99mTcO4 due to the ratio of organ to background activity, ten times higher with 123I. However, the short half-life of this isotope (about 13 h) and the difficult availability explain why it is not of frequent use at the time of diagnosis. In fact, 123I is mainly used nowadays for the differential diagnosis of inborn errors of hormonogenesis in children with an in situ gland, at the time of etiological re-evaluation at 2–3 years old [4]. In these cases a so-called depletion test can be performed. It consists of an intravenous or oral administration of I123 which is followed by a baseline scintigraphic measurement obtained 30 min and 2 h after the injection. When the baseline uptake of 123I is high, a blocker of the organification process, called perchlorate, can be administered immediately after the radioactive iodide. Afterwards, scintigraphic measurements are obtained again 15, 30, 60 and 90 min after the administration of perchlorate. In case of defects in the organification pathway, iodine will be released by the gland after perchlorate. Injectable and oral forms of perchlorate are available. When perchlorate is used, a fall of below 10 % is considered normal, while a fall between 10 and 50 % indicates a mild partial organification defect and a fall between 50 and 90 % indicates a severe partial organification defect. A relative fall of over 90 % indicates a complete organification defect [6] (Fig. 7.3) (Table 7.1).
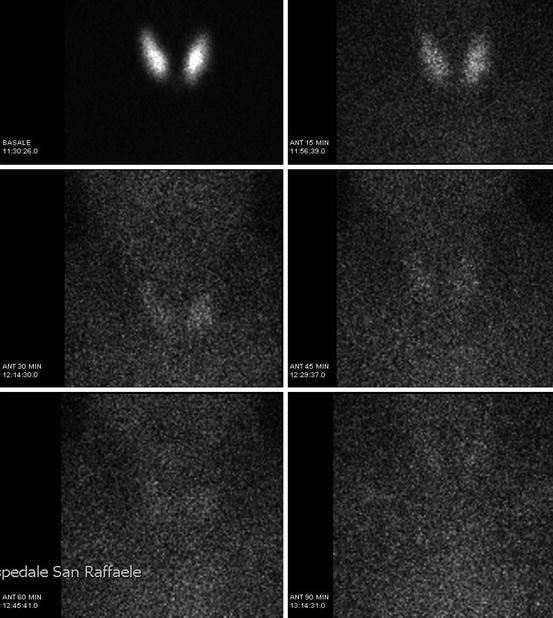
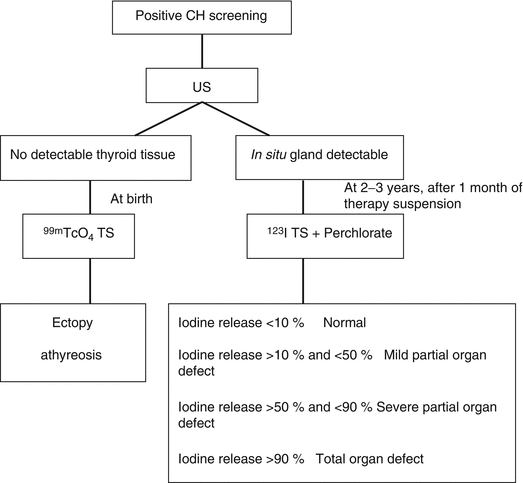

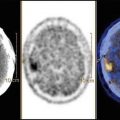
Fig. 7.3
Fall of over 90 % at perchlorate test at etiological re-evaluation
Table 7.1
Uses of scintigraphy in CH

As concerns the dose administered, it is very low with both isotopes. Furthermore, in children with athyrosis, hypoplasia, and defective thyrotropin hormone receptor (RTSH) or sodium/iodide symporter (NIS), the isotopic uptak is very low or absent. The only case where a significant isotopic dose can be described is when the uptake is preserved (ectopic glands, thyroglobulin defect, iodotyrosine dehalogenase defects and in transient forms). However, no reports have been made to describe adverse outcomes in these patients.
Finally, a possible alternative to L-thyroxine withdrawal is to perform a so-called rhTSH test [7]. The test can be performed very early in postnatal life. After the administration of a TSH analogue (rhTSH – Thyrogen), serum TSH, free T4, free T3, thyroglobulin levels and anti-thyroglobulin/anti-thyroperoxidase antibody levels are measured. Furthermore, an ultrasound examination and a thyroid scintigraphy with 123I and a discharge test can be performed. This approach permits to distinguish between permanent and transient forms of CH with an in situ gland, preventing the unnecessary prolongation of replacement treatment in transient CH. However, the diffusion of the test is still limited by the high costs that explain why it is poorly used worldwide.
7.2 Hyperthyroidism
Nuclear medicine is not considered a diagnostic tool in the management of hyperthyroidism, while it has a primary importance in the definitive treatment of this disease. The optimum therapeutic strategy for hyperthyroidism still represents a controversial topic in paediatric endocrinology. Medical therapy, radioactive iodine (RAI) therapy and surgery (thyroidectomy) are the current treatment approaches for Graves’ disease [8]; nevertheless only 30 % of medical treated children achieve remission [9].
Radioactive iodine therapy is a valid treatment strategy in case of refractory hyperthyroidism or relapse of the disease despite thioamides, low compliance with medical therapy and adverse drug reactions [10]. Controversially, while in Europe this treatment is not usually recommended in the prepuberal period, in the USA it is considered a first-line therapy for children >10 years of age and a second-line strategy in patients 5–10 years who failed with medical therapy [8].
RAI therapy’s main goal is to induce hypothyroidism by thyroid ablation: radioactive iodine is trapped by thyrocytes with high affinity, and either gamma or beta radiations, which are responsible for the therapeutic effect, are emanated. Fixed or individually calculated doses of 131I on gland size and on RAI uptake are alternatively used, both with optimal outcomes (Table 7.2). However, large glands (>80 g) should be surgically treated since RAI therapy seems not to be effective [8, 10], and eventually a steroid therapy is the gold standard in case of active ophthalmopathy [10].
Table 7.2
Fixed and individually calculated doses of 131I
Fixed dose | 15 mCI |
Individually calculate dose | >5.5 MBq per gram of thyroid tissue weight |
7.4–11.1 MBq per gram of thyroid tissue, if larger gland (30–80 g) |
Radioactive 131I can be given orally, or rarely intravenously. Patients should not take antithyroid drugs from 3 to 5 days before the treatment to at least 7 days after. Moreover, iodine-containing products (e.g. cream, disinfectant, hair dye, amiodarone, contrast agents) should be avoided in the period before the RAI therapy as a low-iodine diet should be followed. By 2–3 months, patients develop hypothyroidism, while retreatment is indicated if hyperthyroidism persists 4–6 months after the therapy [10].
The main side effects of the treatment are summarised in Table 7.3.
Table 7.3
Side effects
Thyroid tenderness (first week) |
Thyroid storm |
Worsening of ophthalmopathy |
Thyroid cancer/thyroid nodules |
Acetaminophen or nonsteroidal anti-inflammatory agents are useful in case of mild tenderness over the thyroid, which could occur in less than 10 % of the patients [8]. In case of uncontrolled hyperthyroidism (fT4>60pMol/L), it is recommended to normalise fT4 levels before the therapy, in order to avoid a secondary thyroid storm. There is no evidence of association between 131I and genetic damage in the patient, as no cases of thyroid cancer and other tumours have been reported in patients treated with >5.5 MBq of 131I per g of tissue. Therefore, low doses have to be avoided, since there is a higher risk of developing a cancer in presence of residual thyroid tissue especially in young children [10].
7.3 Toxic Adenoma
Toxic adenoma is a hyperfunctioning nodule that results in hyperthyroidism [5]. Amongst the causes of hyperthyroidism it represents a rare one, in particular if compared to the most frequent one, which is Graves’ disease. Toxic adenomas are also referred to as toxic autonomous nodules and Plummer disease and are characterised by an autonomous and continuous production of thyroid hormone that does not respond to the normal hypothalamic-pituitary control mechanisms. Moreover, the excess of thyroid hormone suppresses the further production of hormones in the residual normal gland. Unlike Graves’ disease, the mechanism of toxic adenoma is not autoimmunity. In fact, it is thought that the TSH receptors on the surface of the adenoma undergo gene mutation becoming permanently active.
Adenomas can frequently present with forms of subclinical hyperthyroidism: the affected children show suppressed TSH values, elevated T3/T4 values without antibodies against TSH receptor.
Ultrasound imaging represents the first-line screening tool to detect thyroid nodules. It is fundamental in assessing the number of nodules. US imaging shows a mass lesion with variable features. Echogenicity can be diminished or increased and homogeneous or heterogeneous. Normally they are described as hypovascular.
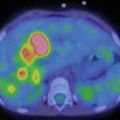
As concerns the scintigraphic findings, children with toxic adenomas will typically show a solitary nodule with a significant radioiodide uptake, also called “hot nodule”, while the rest of the gland shows a diminished uptake (Fig. 7.4). This is due to the negative pituitary feedback on TSH secretion, leading to a decreased or absent tracer uptake.
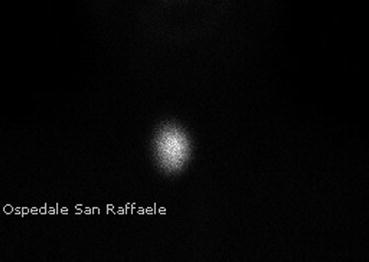

Fig. 7.4
Scintigraphy of a toxic adenoma
These lesions can be treated surgically or with radioiodine. The former is at present the preferred one in childhood, due to the high doses of isotope necessary when using the latter technique.
7.4 Primary Hyperparathyroidism
Ninety percent of hypercalcemia associated to primary hyperparathyroidism cases are due to an adenoma in a single parathyroid. The preoperative localization with ultrasonography and radionuclide scintigraphy allows a selective surgical excision of the hyperfunctionating parathyroid gland [11]. In the past, when preoperative imaging was not used, the unguided bilateral surgery failed in 5–10 % of patients, especially in the presence of an ectopic parathyroid or a multiple gland disease. While the advantages of radioguidance – a reduction in conversion to bilateral exploration, operative time, length of stay and total costs – have been well defined in adults, in paediatric patients only few studies have examined the utility of preoperative localization with radionuclide [12].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree