This article briefly discusses the clinical features, natural history, and epidemiology of intracranial cerebral aneurysms, along with current diagnostic imaging techniques for their detection. The main focus is on the basic techniques used in endovascular coiling of ruptured and nonruptured saccular intracranial cerebral aneurysms. After a discussion of each technique, a short review of the results of each form of treatment is given, concentrating on reported large case series. Specific complications related to the endovascular treatment of saccular intracranial aneurysms are then discussed.
Key points
- •
The annual risk of aneurysm rupture, based on the International Study of Unruptured Intracranial Aneurysms, is 0.7% per year.
- •
The 30-day case fatality after subarachnoid hemorrhage (SAH) is 38%, and 10% to 20% of SAH survivors remain dependent.
- •
Cerebral aneurysms can be treated with simple coiling, balloon remodeling, stent-assisted coiling, Onyx HD 500, and flow diversion.
- •
The International Subarachnoid Trial showed an absolute risk reduction in death of 7.4% in patients presenting with SAH treated with endovascular coiling in comparison with surgical clip placement.
- •
A Barrow Institute study prospectively randomized 500 SAH patients to clipping versus coiling. At 1 year a modified Rankin Score of greater than 2 was observed in 33.7% of clipped patients, compared with 23% of patients with coils.
- •
The Pipeline Embolization Device in the Intracranial Treatment of Aneurysms trial treated 31 intracranial aneurysms with a mean aneurysm size of 11.5 mm and mean neck size of 5.8 mm. Follow-up angiography at 6 months demonstrated complete occlusion of the aneurysm in 93.3% of patients.
Introduction
Microsurgical clipping of intracranial aneurysms has been the historical definitive standard for the treatment of intracranial aneurysms. Today’s surgical techniques routinely achieve complete exclusion of the aneurysm from the circulation without compromise of the parent vessel or arterial perforators in a large number of patients. However, there are several risk factors that may put the patient at increased risk of morbidity and mortality, including aneurysm size and location, patient’s age, and the medical condition of the patient. In addition, according to the International Subarachnoid Trial (ISAT), patients with subarachnoid hemorrhage (SAH) fared better with endovascular coiling than those with surgical clipping. To overcome some of the limitations of surgical clipping, endovascular treatments have been developed, which have grown considerably in number over the last 15 years since the US Food and Drug Administration (FDA) approval of the Guglielmi detachable coil (GDC) in 1995. This article briefly discusses the clinical features, natural history, and epidemiology of intracranial cerebral aneurysms, along with current diagnostic imaging techniques for their detection. The main focus is on the basic techniques used in endovascular coiling of ruptured and nonruptured saccular intracranial cerebral aneurysms. After a discussion of each technique, a short review of the results of each form of treatment is given, concentrating on reported large case series. Specific complications related to the endovascular treatment of saccular intracranial aneurysms are then discussed.
Introduction
Microsurgical clipping of intracranial aneurysms has been the historical definitive standard for the treatment of intracranial aneurysms. Today’s surgical techniques routinely achieve complete exclusion of the aneurysm from the circulation without compromise of the parent vessel or arterial perforators in a large number of patients. However, there are several risk factors that may put the patient at increased risk of morbidity and mortality, including aneurysm size and location, patient’s age, and the medical condition of the patient. In addition, according to the International Subarachnoid Trial (ISAT), patients with subarachnoid hemorrhage (SAH) fared better with endovascular coiling than those with surgical clipping. To overcome some of the limitations of surgical clipping, endovascular treatments have been developed, which have grown considerably in number over the last 15 years since the US Food and Drug Administration (FDA) approval of the Guglielmi detachable coil (GDC) in 1995. This article briefly discusses the clinical features, natural history, and epidemiology of intracranial cerebral aneurysms, along with current diagnostic imaging techniques for their detection. The main focus is on the basic techniques used in endovascular coiling of ruptured and nonruptured saccular intracranial cerebral aneurysms. After a discussion of each technique, a short review of the results of each form of treatment is given, concentrating on reported large case series. Specific complications related to the endovascular treatment of saccular intracranial aneurysms are then discussed.
Clinical features
Most intracranial aneurysms are asymptomatic and remain undetected until the time of rupture. Autopsy reports have shown that aneurysms are present in roughly 5% of the population. SAH, a medical emergency, is the most common initial clinical presentation. It was the first symptom in 58% of patients in one series. A history of the abrupt onset of a severe headache of atypical quality (“the worst headache of my life”) is typical of SAH; this may be associated with brief loss of consciousness, a focal neurologic deficit, nausea and vomiting, or meningismus. However, SAH is frequently misdiagnosed. Nearly one-half of the patients present with a milder form of a headache 1 to 2 weeks before full rupture of the aneurysm. A review of 111 patients referred to a tertiary care center for the management of unruptured aneurysms found that only 41% of the aneurysms produced symptoms.
Common presentations of intracranial aneurysms in specific locations include the following.
Paraclinoid Aneurysms
Paraclinoid aneurysms, like other intracranial aneurysms, present with SAH. The second most common form of presentation for aneurysms in this location is visual loss, which is usually unilateral from involvement of the ipsilateral optic nerve. As an ophthalmic artery aneurysm grows, it usually impinges on the lateral aspect of the optic nerve. This process can produce a monocular superior nasal quadrantanopia, and in an aneurysm with significant mass effect of the nerve complete visual loss may be present. A much more unusual form of presentation is that of ischemic symptoms caused by embolization from a partially thrombosed aneurysm. A majority of patients presenting with a paraclinoid aneurysm are women, and there is a high incidence of multiple aneurysms in patients with aneurysms in this region. Posterior communicating aneurysms usually present with SAH or isolated third-nerve palsy. The third-nerve paresis is commonly painful and the pupil is usually involved. Anterior choroidal artery aneurysms can on occasion present with a third-nerve palsy.
Aneurysms of the Internal Carotid Artery Bifurcation
Aneurysms arising at the distal bifurcation of the internal carotid artery also commonly present with SAH. These aneurysms can produce a large intraparenchymal hematoma that may be mistaken for a hypertensive hemorrhage. The lesions may also cause signs and symptoms owing to a local mass effect.
Anterior Communicating Artery Aneurysms
Aneurysms in this location typically present with SAH. The initial hemorrhage may be minor, causing a severe headache without neurologic signs, or may be more extensive, causing a neurologic deficit. Given the intimate location of these aneurysms within the third ventricle and hypothalamus, local hemorrhage often causes changes in mental status including confusion, memory distortion, or even coma. The aneurysm commonly ruptures into the third ventricle, often causing hydrocephalus. Involvement of the hypothalamic region can produce bradycardia, diabetes insipidus, inappropriate antidiuretic hormone secretion, and visual disturbances. Rarely there is bleeding into the internal capsule, causing hemiparesis.
Middle Cerebral Artery Aneurysms
The most common presentation for patients with a middle cerebral artery aneurysm is SAH. The second most common is incidental detection of an asymptomatic aneurysm on computed tomography (CT) or magnetic resonance (MR) imaging. Large middle cerebral artery aneurysms can cause local mass effect on the temporal lobe, leading to a seizure. Transient ischemic attacks and cerebral infarction are uncommon with intracranial aneurysms, although they are more frequent in this location. About 60% of patients with a middle cerebral artery aneurysm will have a transient loss of consciousness at the time of rupture. About one-third of patients will have a headache localized to the side of the aneurysm. Middle cerebral artery aneurysms are more likely than an aneurysm in any other location to present with an intraparenchymal hematoma. In addition, a patient with a ruptured middle cerebral artery aneurysm is more likely to have a subdural hematoma.
Basilar and Posterior Cerebral Artery Aneurysms
Patients with basilar and posterior cerebral artery aneurysms commonly present with SAH indistinguishable from SAH from other aneurysms. Occasionally large or giant aneurysms in this location will present with seizures, hydrocephalus, or signs of mass effect such as hemiparesis or third-nerve palsy.
Vertebrobasilar Junction Aneurysms
Aneurysms of the vertebrobasilar junction usually arise from the carina between the basilar artery and the dominant vertebral artery. Occasionally they occur in relation to a fenestration of the lower basilar artery. The patient usually presents with SAH or signs of lower brainstem compression.
Posterior Inferior Cerebellar Artery Aneurysms
Aneurysms of the vertebral-posterior inferior cerebellar artery usually arise at the junction of both of these vessels, and have a tendency to point superiorly and lie against the medulla. The aneurysm usually has a close relationship with the vagal and hypoglossal nerves. SAH hemorrhage is the most common presentation, often accompanied by a palsy of the sixth or lower cranial nerves. Headache is usually localized to the neck and occipital region.
Epidemiology and natural history
The precise prevalence of intracranial aneurysms is not known and varies widely in the literature. It is accepted that about 3% to 5% of the population should harbor an intracranial aneurysm. The frequency of intracranial aneurysms in adult autopsy studies ranges from 1% to 6%. According to angiographic series, the incidence of intracranial aneurysms varies from 0.65% to 7%. Berry aneurysms are multiple in about 20% to 30% of cases. Intracranial aneurysms are also more prevalent in females, and are lesions of adulthood with the peak prevalence in the fourth and sixth decades. Aneurysms in the pediatric population are rare, accounting for 2% to 4% of all aneurysms; they are often are found in males, and tend to be larger and of the dissecting variety.
Several medical conditions are associated with the development of intracranial aneurysms, including systemic lupus erythematosus, Takayasu disease, giant cell arteritis, autosomal polycystic kidney disease, type IV Ehlers-Danlos syndrome, Marfan syndrome, fibromuscular dysplasia, type 1 neurofibromatosis, pseudoxanthoma elasticum, hereditary hemorrhagic telangiectasia, coarctation of the aorta, and α1-antitrypsin.
Genetics also plays a role in the formation of intracranial aneurysms. If 2 first-degree relatives in the same family have an intracranial aneurysm with no heritable connective tissue disorder, immediate family members have up to a 17% incidence of harboring an unruptured aneurysm. Furthermore, first-degree and second-degree relatives of a patient with aneurysmal SAH are at 4.1- to 6.6-fold increased risk for aneurysmal SAH. Therefore, screening is suggested in patients who have 2 or more family members with intracranial aneurysms.
Other risk factors for the development of an intracranial aneurysm formation include cigarette smoking, cocaine use, and heavy consumption of alcohol. Infections from bacterial or fungal colonization of vessel walls, head trauma, and intracranial neoplasms or neoplastic emboli are rare causes of intracranial aneurysms.
The predominant location for saccular intracranial aneurysms is the anterior circulation (90%), with most arising from the circle of Willis. The anterior communicating complex (30%–35%) is the most common location, followed by the internal carotid artery (30%), including the carotid bifurcation, posterior communicating artery, and ophthalmic artery. The next most common location is the middle cerebral artery bifurcation (20%), followed by the basilar apex (5%), superior cerebellar artery (3%), inferior cerebellar artery (3%), and the vertebrobasilar junction (2%).
The natural history of unruptured intracranial aneurysm is uncertain and is surrounded by controversy. The most known studies on the subject include a comprehensive cohort study (2000) with a patient follow-up of 18 years, a large meta-analysis (1998) that included studies from 1955 to 1966, a systematic review of literature from Japan (2005), and the International Study of Unruptured Intracranial Aneurysms (ISUIA) (part 1, 1998 and part 2, 2003), which compromise the majority of this discussion. Prior studies have extrapolated results from patients with small unruptured intracranial aneurysms (UIAs) diagnosed after SAH to infer that these aneurysms are at a considerable increased risk of rupture. Other studies have used SAH incidence rates to infer prevalence of UIAs in the general population. However, it is important to distinguish UIAs from ruptured intracranial aneurysms, as these separate entities are managed differently. Therefore, the goal in management of UIAs is to identify which UIAs have the greatest risk of rupture and rerupture after treatment.
Results from the 1998 meta-analysis demonstrated an overall 2% prevalence of UIA in the population, with the vast majority of aneurysms being small (10 mm), having an estimated annual risk of rupture of approximately 0.7%. The male/female ratio was approximately 1:1.3 and incidence of an aneurysm increased with age, peaking in the 60- to 79-year-old age group. The main limitation of the study was that the meta-analysis included retrospective studies and was performed before the CT era.
The cohort study published in 2000, performed in Finland from 1956 to 1978, consisted of 142 patients with a mean follow-up of 18 years. Results from the study included an overall frequency of 1.0% UIA with no history of SAH and 1.3% UIA with history of SAH, 2.6% of aneurysms being symptomatic. Frequency of aneurysm by size was 1.1% (2–6 mm), 2.3% (7–9 mm), and 2.8% (10–26 mm). The mortality rate with rupture was 52%. Limitations of the study included lack of power of the test (N = 142), a homogeneous population, and 92% of the patients having a history of SAH.
The 2005 meta-analysis of 13 Japanese studies (3801 patient-years) demonstrated rupture in 104 patients with the annual rupture rate of 2.7% (95% confidence interval [CI] 2.2%–3.3%). Large, posterior circulation, and symptomatic aneurysms were associated with significantly higher rates of rupture (relative risk 6.4, 2.3, and 2.1, respectively). The risk of rupture was significantly higher for the Japanese population when compared with results reported by investigators from international cohort studies. Limitations of the study included retrospective studies and a homogeneous population (single ethnic group).
The ISUIA is the largest study evaluating the history of UIAs (N = 4060) at 53 international centers including Canada, Europe, and the United States. Data acquired from the ISUIA, a large, comprehensive multicenter study with retrospective and prospective cohorts, analyzed the natural history of UIA as well as the morbidity and mortality associated with rupture. In addition, subgroup analysis was performed according to aneurysmal size, aneurysmal location, and history of SAH from a separate aneurysm. The patients were divided into 2 groups. Group 1 included patients with unruptured intracranial aneurysm and no prior SAH, and group 2 consisted of patients with UIA and prior SAH. Overall, rupture rates with UIA smaller than 7 mm were statistically significantly higher ( P <.01) in group 2 when compared with group 1. The site distribution of UIA in patients with no prior history of SAH was 16.9% for cavernous carotid artery, 24.8% for internal carotid artery, 10.0% for anterior cerebral artery and anterior communicating artery, 22.7% for middle cerebral artery, 13.9% for posterior communicating artery, 6.6% for vertebrobasilar or posterior cerebral artery, and 5.1% for basilar artery apex.
In the retrospective cohort, rupture rate was 0.1% per year for patients in group 1 who had UIAs smaller than 10 mm. The rupture rate for aneurysms larger than 10 mm was 1%, and 6% for aneurysms larger than 25 mm. Although aneurysm size was the best predictor, aneurysm location also predicted risk of rupture, with the posterior communicating artery and vertebrobasilar circulation aneurysms more likely to rupture. The rupture rates for the prospective cohorts were higher for aneurysms larger than 7 mm. Averages calculated for predetermined 3-mm successive size categories used in the retrospective cohort allowed the establishment of well-defined cutoff points to less than 7 mm, 7 to 12 mm, 13 to 24 mm, and greater than 25 mm, with each category having statistically significant rates of rupture that allowed for the establishment of prediction models. The 5-year cumulative rate for rupture in group 1 patients with aneurysms in the anterior circulation, excluding cavernous and posterior communicating arteries, was 0 for aneurysms smaller than 7 mm, 2.6 for aneurysms 7 to 12 mm, and 14.5 for aneurysms 13 to 24 mm in size, compared with 2.5, 14.5, and 18.4, respectively for aneurysms in the posterior circulation.
In the retrospective cohort, group 2 rupture rates were 11 times higher than rupture rates in group 1 for UIAs smaller than 10 mm of the same size. Therefore, size alone was not a clear predictor of future risk of rupture. The only clear predictor of the future risk of rupture was aneurysmal location. In the prospective cohort, UIA rupture rates were statistically higher in group 2 with aneurysms smaller than 7 mm, which is of high relevance because a considerable portion of group 2 patients had UIAs smaller than 7 mm. Otherwise, the pattern and rupture rates for UIAs in the group 2 retrospective and prospective cohorts were almost the same.
There is some controversy regarding the ISUIA results because there is an apparent discrepancy between the low rupture rates in the study and the number of SAHs observed in clinical practice. Critics state that the study suffers from selection and intervention bias because most aneurysms observed in practice are 6 mm to 7 mm, which falls within the benign natural history of the study, and the annual prevalence of UIA (2%) does not match the average rupture rates (0.7%) found in the study.
The incidence of aneurysmal SAH is approximately 10 in 100,000 with 21,000 to 33,000 new cases occurring in the United States each year. Women have an increased risk when compared with men and African Americans (1.6×), and Hispanics (1.3×) have greater risks in comparison with Caucasians. The mean age of presentation is 55 years. SAH accounts for 4.4% of stroke mortality and 27.3% of all stroke-related years of potential life lost before age 65. The in-hospital mortality rate is 26.3% and the 30-day case-fatality rate is 38%. Prospective studies, which include deaths occurring before hospital admission, have shown consistently higher mortality rates, ranging from 56% to 86%, with 61% of deaths occurring within 48 hours and most deaths within 2 weeks. Patients with posterior circulation aneurysms are 3 times more likely to die before reaching the hospital or within 48 hours when compared with those with anterior circulation SAH, and 10% to 20% of SAH survivors remain dependent.
Associated Complications of Aneurysmal SAH
Associated complications of aneurysmal SAH include rehemorrhage, hydrocephalus, and seizures. The peak rate of rebleeding occurs within the first 24 hours and is as high as 19%, 20% in the first 2 weeks, and 40% at 1 month, with a mortality rate of up to 74%. Approximately 20% of patients experience ventricular enlargement after aneurysmal SAH. Risk factors for hydrocephalus include subarachnoid and intraventricular hemorrhage; older age of the patient, and posterior circulation aneurysm. Studies have demonstrated that seizures can occur in approximately 8% of patients, with 90% occurring in the first 24 hours after aneurysmal SAH. Multisystemic medical complications are associated with high morbidity and mortality. Approximately 40% of patients have a life-threatening medical complication, and 23% of deaths can be attributed to a medical complication. Hyperglycemia occurs in 30% of patients and is an independent predictor of poor outcome. Hyponatremia occurs in 30% to 43% of patients, and has a strong association with vasospasm. Morinaga and colleagues demonstrated that as much as 84% of patients with hyponatremia had symptomatic vasospasm. Cardiopulmonary complications can occur in patients with aneurysmal SAH and may be related to sympathetic system activation. Serious cardiac arrhythmias occur in 1% to 4% and malignant arrhythmias in up to 4%. Reversible cardiomyopathy (stunned myocardium) and left ventricular dysfunction has been reported in about 10% of cases. The incidence of neurogenic pulmonary edema has been reported at 20% to 27%. Of all medical complications, the leading cause of mortality and morbidity is vasospasm in patients with aneurysmal SAH. Prior studies have demonstrated that varying degrees of vasospasm occur in 70% of patients and is symptomatic (clinical vasospasm) in 20% to 25% of patients. Risk factors for vasospasm include amount of intracranial hemorrhage, age (<50 years), hyperglycemia, hyponatremia, hypertension, aneurysm size, and cocaine use. Therefore, optimal medical management is of paramount importance and, as a result, hyperdynamic therapy (triple-H [permissive hypertension, hypervolemia, and hemodilution]) has become the first-line therapy for patients who have undergone treatment of ruptured aneurysmal SAH.
Diagnostic imaging
Current neuroimaging techniques for intracranial aneurysms include intra-arterial 3-dimensional (3D) digital subtraction angiography (DSA), intra-arterial DSA, multislice computed tomographic (MSCT) angiography, CT angiography with a dual-source CT scanner, MR angiography, and transcranial Doppler ultrasonography. Intra-arterial DSA is still the gold standard for evaluation of a patient with a suspected aneurysm, given its high spatial resolution; however, currently MSCTA has a similar specificity and sensitivity for detecting an intracranial aneurysm, except for aneurysm smaller than 3 mm. The spatial resolution for DSA is 0.2 mm, and about 0.15 mm for 3D-DSA. Intra-arterial DSA is an invasive test with a 1% risk of transient neurologic complications and a 0.5% risk of permanent neurologic complications, whereas there is no risk of neurologic complications with MSCTA or MR angiography.
To a large extent, sensitivity for detection of aneurysms depends on spatial resolution, which itself is dependent on the number of detector rows available on a helical CT scanner. The highest current spatial resolution of CT angiography is 0.4 to 0.7 mm on a 64-MSCT scanner. When compared with DSA as the reference standard, CT angiography has an average reported specificity rate of 96% to 98% (90% to 94% for aneurysms smaller than 3 mm and up to 100% for aneurysms larger than 4 mm) and sensitivity rates of 96% to 98%. With the advent of multidetector CT scanners with a very high number of detectors (eg, 256-slice and 320-detector row scanners), further improvements in spatial resolution and accuracy are expected.
The spatial resolution of MR angiography at 1.5 T is on the order of 1 mm. With 3-T scanners, spatial resolution may reach 0.6 mm. When compared with DSA, MR angiography reported specificity rates for detection of intracranial aneurysms ranging from 69% to 100%, with a sensitivity rate of 75% to 100%.
Treatment
Conventional Coiling of a Simple Saccular Aneurysm
Technique
At the authors’ institution, all intracranial aneurysm coiling procedures are performed under general anesthesia with neurologic monitoring. An arterial line is placed in the radial artery to closely monitor the patient’s blood pressure. The anesthesiologist is aware of the need to avoid transient blood pressure spikes, especially when intubating or extubating the patient; this is particularly important in a patient who has a ruptured aneurysm. The procedure can sometimes take a few hours, therefore anesthesia is necessary to maintain immobility because 3D imaging and a fluoroscopic roadmap is very motion sensitive. A 6F sheath is inserted into the right common femoral artery. If balloon remodeling or additional microcatheters are needed, puncture of the left common femoral artery may be necessary. Just after the sheath is inserted in the groin, a baseline activated clotting time (ACT) is drawn. Sixty to 100 international units (IU) of heparin per kilogram are then given intravenously as a bolus. For patients with unruptured aneurysms the ACT is checked every 30 minutes throughout the procedure, and heparin is given intermittently to keep the ACT between 250 and 300 seconds. For patients with a ruptured aneurysm, 3000 IU of heparin is given initially, and after placement of framing coils the patient is then given additional heparin to maintain an ACT range of 250 to 300. A syringe of protamine is prepared in advance and is readily available to be injected in case of aneurysm rupture. The usual dose is 10 mg of protamine per 1000 IU of heparin. After the procedure, the heparin is reversed, and hemostasis can be obtained with manual compression if the ACT is less than 200 seconds. Likewise, for patients with unruptured aneurysms who have been treated preoperatively with dual antiplatelet therapy, a 5-pack of unpooled platelets is kept in preparation in case of rupture.
Patients who have not suffered a recent SAH are preoperatively given 75 mg clopidogrel and 81 mg aspirin orally, starting 7 days before the procedure, to prevent thromboembolic complications. If emergent platelet inhibition is needed, the patient is loaded with 600 mg clopidogrel and 325 mg aspirin. Full platelet inhibition occurs 2 hours afterward. If the procedure needs to be performed urgently, the patient may be given a glycoprotein IIa/IIIb inhibitor. A platelet inhibition assay is drawn for clopidogrel and aspirin before treatment, because approximately 25% of patients will have clopidogrel or aspirin resistance.
A complete cerebral angiogram is performed before treatment with a 4F or 5F catheter, including injections of the bilateral common carotid, internal carotid, and vertebral arteries. Additional 3D rotational images are obtained to more accurately define the neck, dome, and size of the aneurysm. Once the diagnostic portion of the procedure is performed the catheter is exchanged for a 6F guide catheter, which is positioned as close as possible to the aneurysm. It is necessary for the guide catheter to have a stable position so as to be able to introduce a microcatheter safely into the aneurysm. For this reason, the guide catheter should be positioned as close to the aneurysm as possible. This positioning gives optimal stability of the guide catheter and allows the operator to monitor the guide position on the same roadmap as the microcatheter during advancement of the coil into the aneurysm. New guide catheters that are more flexible, such as the Neuron (Penumbra, Alameda, CA), Navien (eV3, Irvine, CA), or distal accesses catheters (DAC) (Stryker, Fremont, CA) can be introduced further into the intracranial circulation, and can be routinely placed in the cavernous internal carotid artery or the distal vertebral artery. This placement allows more stability in advancing devices into the intracranial circulation. This increased stability has been a major innovation in the endovascular treatment of aneurysms over the last 3 years. If additional stability is needed to treat an aneurysm, a triple coaxial system consisting of a long sheath introduced into the origin of the great vessel followed by a guide catheter through this may offer enhanced stability in advancing devices through tortuous anatomy for the treatment of intracranial aneurysms. If an aneurysm cannot be treated from a femoral artery approach, a brachial, radial, direct carotid or vertebral artery puncture is another option.
Microcatheter placement into the aneurysm
All microcatheters have an outer hydrophilic coating; this reduces the friction between the catheter and inner wall of the blood vessel, thus facilitating distal catheterization of the intracranial circulation. The microcatheter is advanced over a microwire into the aneurysm under roadmap. The microcatheter is never advanced into the aneurysm without using a wire, because this may perforate the aneurysm. Once the microcatheter is positioned within the aneurysm, the slack within the system is carefully removed and the wire slowly withdrawn to prevent forward movement of the microcatheter tip. The best microcatheter position depends on the size and shape of the aneurysm; however, the best position is usually in the middle of the aneurysm or at the origin of the neck. This configuration usually allows the coil to form within the aneurysm with minimal resistance. Some operators in large aneurysms will wrap the microcatheter around the dome of the aneurysm and place the tip of the catheter at the neck. These surgeons claim that this allows for placement of more coil loops across the neck of the aneurysm, thus limiting the possibility of prolapse of additional coils into the parent vessel during filling of the aneurysm.
Coil selection
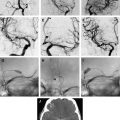
The choice of the coil is based on the size and shape of the aneurysm as seen on the angiogram and 3D reconstruction. The purpose of the first coil (framing coil or complex shape coil) is to create a support basket for the subsequent introduction of additional coils, and to provide a bridge to prevent additional coils from migrating into the parent vessel. The first coil is sized to the largest dimension of the aneurysm sac. The first coil should be as large and long as possible to fully appose the aneurysm wall, and provide a sound basket and stability to prevent the coil from prolapsing into the parent vessel. This maneuver will also provide the maximum number of coil loops across the neck of the aneurysm, thus preventing herniation of additional coils placed into the aneurysm from compromising the parent vessel ( Fig. 1 ).
If the aneurysm sac has a sausage-like appearance, the principle guiding the selection of the first coil is different. In such aneurysms, a helical coil is preferred to a complex-shaped coil, and the first coil is sized to the smallest sac diameter. The subsequent coils are similar in size and length, with the aneurysm coiled from the dome toward the neck.
Coil placement
Through the microcatheter, the platinum portion of the coil is introduced into the aneurysm sac while still on the delivery wire. In the microcatheter, the coil assumes a straight configuration; however, as soon as the coil leaves the microcatheter it assumes the manufactured memory shape. The coil is radiopaque under fluoroscopy and is visualized under live roadmap as it is placed into the aneurysm sac with the neck in profile. There should be limited resistance when introducing the coil into the aneurysm sac. If there is any resistance, the coil maybe oversized. It is important not to force the coil into the aneurysm sac, to prevent possible rupture. If the coil is correctly sized it will readily adapt to the shape of the aneurysm. If the coil is undersized, it will not be stable within the aneurysm sac and may herniate into the parent vessel. If the coil is oversized, it will not form with the aneurysm sac and will herniate into the parent vessel. Oversizing the coil may also cause excessive pressure on the wall of the aneurysm sac, possibly risking perforation.
Before detachment of the coil, an angiogram is performed to determine how the coil is confined within the aneurysm sac and to determine if there is compromise of the parent vessel. If there is movement of the coil during the angiogram, this may indicate that the coil is undersized and may migrate out of the aneurysm sac after detachment. If the coil appears properly sized and there is no compromise of the parent vessel, the coil is detached under fluoroscopy. The detachment wire is then slowly pulled back through the microcatheter to make sure the coil has detached from the wire and to ensure that the coil does not move.
Additional coils of various sizes and shapes are subsequently introduced into the aneurysm sac until the sac is densely packed and no longer filling with contrast or the microcatheter is pushed outside the sac. The first coils used for the treatment of intracranial aneurysms were made of platinum; however, there were aneurysm recurrences after treatment, so bioactive coils were introduced with the goal of inducing an exuberant healing response and improved filling volume of the coiled aneurysm. The first bioactive coil was the Matrix (Stryker, Fremont, CA), introduced in 2002. The FDA approved the Matrix coil based on equivalency with the conventional GDC coil. Four bioactive coils are now available for clinical use: Matrix, Hydrocoil (Microvention, Tustin, CA), Cerecyte (Codman Neurovascular, Raynham, MA), and Axium (Ev3, Irvine, CA). The newer coils are manufactured so that the aneurysm sac is filled in a Russian nesting doll manner from the periphery toward the center. Filling coils are placed into the aneurysm sac after the placement of framing coils; once the aneurysm is nearly densely packed, the final coils usually placed into the aneurysm sac are finishing coils. These coils are very short and soft. Once the aneurysm is densely packed the microcatheter is removed slowly from the aneurysm, and a posttreatment angiogram is performed to assess the degree of aneurysm occlusion, the parent vessel, and the patency of the distal vasculature.
The heparin is reversed after the procedure with protamine, and manual pressure or a closure device is used to obtain hemostasis at the femoral puncture site. Pressure is usually held on the puncture site for 20 to 30 minutes after removal of the sheath if manual compression is used, and the leg immobolized for 6 hours to prevent groin complications. If a closure device is used, ambulation can occur as early as 2 hours after placement. If there is compromise of the parent vessel, protrusion of coils, or thrombus formation during the coiling procedure, the heparin may be continued overnight with the sheath left in place. Antiplatelet therapy may also be given.
After endovascular treatment of nonruptured intracranial aneurysms, the patient is kept in the neurointensive care unit (NICU) for at least 24 hours for close monitoring of blood pressure, neurologic status, and puncture site. SAH patients are kept in the NICU for at least 14 days under close neuromonitoring and are prophylactically treated for vasospasm.
Results of endovascular treatment of ruptured aneurysms
Three prospective randomized trials have compared outcomes of endovascular coiling versus surgical clipping.
The first study was performed in Finland, and randomized 109 patients with SAH who were suitable for either surgery or endovascular coiling. Angiographic outcome in the posterior circulation was significantly better for endovascular coiling, whereas angiographic outcome in the anterior circulation was significantly better for surgery. Angiographic outcomes in the internal carotid artery and middle cerebral artery were similar in both groups. Glasgow Outcome Scale scores were equivalent in both groups at 3 months. Mortality for technical reasons during surgery was twice that of the endovascular group (4% vs 2%). One patient in the endovascular group suffered rebleeding following incomplete coiling of the aneurysm.
The second study was the ISAT, in which nearly 2000 patients with SAH from predominantly Europe were randomized to surgery or endovascular coiling based on judgment of the treating team. Outcomes analysis on the basis of death or dependence at 2 months and 1 year based on the Modified Rankin score (mRS) was the primary parameter of interest in the first 2002 publication. At 1 year postprocedure, 250 of 1063 (23.5%) of the endovascular patients were dead or dependent while 326 of 1055 (30.9%) of the surgical patients had died. This figure represents an absolute risk reduction of 7.4% for those patients’ treated from an endovascular approach. Delayed rebleeding was more common in the endovascular group; however, several of the cases were due to incomplete treatments. Seizures were also less common in the endovascular group. In the most recent follow-up report there was an increased risk of recurrent bleeding from a coiled aneurysm in comparison with a clipped aneurysm, but the risk was small. The risk of death at 5 years was significantly lower in the coiled group than in the clipped group. Scott and colleagues showed improved cognitive outcomes with endovascular coiling of ruptured intracranial aneurysms from the ISAT trial.
A third study performed at the Barrow Institute examined the null hypothesis that no difference exists between microsurgical clipping and endovascular coil embolization for acutely ruptured cerebral aneurysms. The investigators screened 725 patients with SAH, resulting in 500 eligible patients who were enrolled prospectively in the study. Patients were assigned in an alternating fashion to surgical aneurysm clipping or endovascular coil embolization. Two hundred thirty-eight patients were assigned to aneurysm clipping and 233 to coil embolization. The 2 groups were well matched. One year after treatment, 403 patients were available for evaluation and of these, 358 patients had actually undergone treatment. The remainder either died before treatment or had no identifiable source of SAH. A poor outcome (mRS >2) was observed in 33.7% of the patients assigned to aneurysm clipping and in 23.2% of the patients assigned to coil embolization (odds ratio 1.68, 95% CI 1.08–2.61; P = .02). No patient treated by coil embolization suffered a recurrent hemorrhage. The conclusion of the study 1 year after treatment favored coil embolization.
The CLARITY trial was a prospective, multicenter, consecutive series that compared patients treated with GDC or Matrix coils for ruptured aneurysms. Five hundred seventeen patients harboring aneurysms were treated with GDC (276 patients) or Matrix coils (241 patients). Postoperative and midterm anatomic results were evaluated blindly using the Modified Montreal Scale (complete occlusion, neck remnant, and aneurysm remnant). At the midterm follow-up (mean 16.7 months in the GDC group and 15.4 months in the Matrix group), complete occlusion was reported in 95 of 276 aneurysms (34.4%) in the GDC group and 80 of 241 (33.2%) in the Matrix group; neck remnant in 127 of 276 (46.0%) in the GDC group and 118 of 241 (49.0%) in the Matrix group; and aneurysm remnant in 54 of 276 (19.6%) in the GDC group and 43 of 241 (17.8%) in the Matrix group. Aneurysm occlusion was improvement in 35 of 272 aneurysms (12.9%) in the GDC group and 27 of 239 (11.3%) in the Matrix group; stable situation in 98 of 272 (36.0%) in the GDC group and 97 of 239 (40.6%) in the Matrix group; and worsening in 139 of 272 (51.1%) in the GDC group and 115 of 239 (48.1%) in the Matrix group. A total of 32 out of 517 patients were retreated during the follow-up period: 9 of 276 (3.3%) in the GDC group and 23 of 241 (9.5%) in the Matrix group ( P = .003). The study concluded that the midterm anatomic results and evolution of aneurysm occlusion were not different in patients with ruptured aneurysms treated with GDC or Matrix coils.
The CONSCIOUS-1 trial showed that aneurysm coiling was associated with less angiographic vasospasm and delayed ischemic neurologic deficit than was surgical clipping, whereas no effect on cerebral infarction or clinical outcome was observed in patients presenting with SAH.
Results of endovascular treatment of unruptured aneurysms
The data from endovascular treatment versus surgical clipping for unruptured aneurysms does not show a clear benefit for one form of treatment over the other. A review of modern large clipping and coiling trials for unruptured aneurysms was published in 2005. A majority of these trials were nonrandomized and retrospective. Adverse outcomes were estimated at 8.8% for endovascular coiling and 17.8% for clipping. In the International Study of Unruptured Intracranial Aneurysm (USUIA), adverse outcomes were less common with endovascular treatment (9.3%) than with surgery (13.7%). However, the study was nonrandomized, and the endovascular treatment group included a higher number of elderly patients, larger aneurysms, and aneurysms within the posterior circulation. Surgical adverse outcomes in the USUIA study correlated with patient age greater than 50 years, aneurysm size greater than 12 mm, location in the posterior circulation, previous ischemic cerebrovascular disease, and symptoms of mass effect from the aneurysm. Endovascular outcomes were less influenced by these factors.
The Analysis of Treatment by Endovascular approach of Nonruptured Aneurysms (ATENA), published by Pierot and colleagues, has been one of the most comprehensive studies on the treatment of unruptured aneurysms. The study included 649 patients harboring 739 aneurysms. Seven hundred twenty-seven aneurysms were treated using coils (98.4%). Coils alone were used in 396 aneurysms (54.4%), with balloon remodeling in 271 cases (37.3%), intracranial stents in 57 cases (7.8%), and the Trispan neck bridge in 3 cases (0.4%). Initial results indicated that postoperative occlusion was complete in 436 aneurysms (59.0%), with neck remnant in 160 aneurysms (21.7%) and aneurysm remnant in 143 aneurysms (19.3%). Additional trials on unruptured aneurysms are needed.
Results of endovascular treatment of ruptured and unruptured aneurysms
The Hydrocoil Endovascular Aneurysm Occlusion and Packing Study (HELPS) trial compared hydrogel-coated coils with bare platinum coils for the endovascular treatment of intracranial aneurysms. This randomized controlled trial was performed in 24 centers in 7 countries. Patients with a previously untreated ruptured or unruptured cerebral aneurysm of 2 to 25 mm in maximum diameter were randomly allocated (1:1) to aneurysm coiling with either hydrogel-coated coils or standard bare platinum coils. Primary outcome was a composite of angiographic and clinical outcome at 18-month follow-up. Two hundred forty-nine patients were allocated to the hydrogel coil group and 250 to the control group. Seventy (28%) patients in the hydrogel group and 90 (36%) control patients had an adverse composite primary outcome, giving an absolute reduction in the proportion of adverse composite primary outcomes with hydrogel of 7.0% (95% CI 1.6–15.5) and odds ratio (OR) of 0.73 (95% CI 0.49–1.1, P = .13). In subgroup analysis of recently ruptured aneurysms, there were more adverse composite primary outcomes in the control group than in the hydrogel group (OR 2.08, 95% CI 1.24–3.46; P = .014). There were 8.6% fewer major angiographic recurrences in patients allocated to hydrogel coils (OR 0.7, 95% CI 0.4–1.0; P = .049). The study was inconclusive as to whether hydrogel coils reduce late aneurysm rupture or improve long-term clinical outcome, but their use does lower major recurrence.
The Cerecyte coil trial evaluated the procedural safety and clinical outcomes in patients with ruptured and UIA. Five hundred patients with a ruptured or unruptured aneurysm were randomized to receive Cerecyte or bare platinum coils. Two hundred forty-nine patients were allocated to Cerecyte coils and 251 to bare platinum coils. There was a statistical excess of poor outcomes in the Cerecyte arm at discharge in the ruptured aneurysm groups and at 6-month follow-up in the unruptured group.
The Matrix and Platinum Science (MAPS) trial was recently presented in abstract form at several national meetings. Trial results demonstrated that overall, Matrix 2 detachable coils are as effective as GDC detachable coils with target aneurysm recurrence (TAR) rates of 13.3% versus 14.6%, respectively. In aneurysms with good occlusion immediately postprocedure, Matrix 2 detachable coils demonstrated a statistically significant, superior long-term TAR rate of 2.7% compared with the GDC detachable coils’ rate of 9.6%.
Coiling of Wide-Necked Aneurysms
Balloon remodeling technique
Side-wall wide-necked aneurysm
The main feature that limits the endovascular treatment of aneurysms is the width of the neck. Other features that may limit treatment include the shape of the aneurysm. In 1992, Moret and colleagues introduced the balloon remodeling technique for the treatment of wide-necked intracranial aneurysms. The technique involves placing a nondetachable balloon across the neck of the aneurysm during each coil placement. The coils remain molded around the balloon after deflation of the balloon, essentially “remodeling the arterial wall.” The technique has been improved over the last 17 years with better coils and balloons. It is routinely used today to treat wide-necked aneurysms particularly in patients with SAH, thus eliminating stent placement and the use of antiplatelet agents. Most interventionists consider an aneurysm neck to be wide when the ratio between the maximum diameter of the aneurysm sac and the size of the neck is 1 or less.
To treat wide-necked side-wall aneurysms not at an arterial bifurcation, the authors routinely use the over-the-wire compliant HyperGlide balloon (Ev3, Irvine, CA). If distal catheterization of the neck of the aneurysm is difficult they have used the less compliant gateway balloon (Stryker, Fremont, CA) over a more torquable Synchro 14 wire (Boston Stryker, Fremont, CA) to perform balloon remodeling.
The authors prefer to perform the procedure using a 6F shuttle sheath (Cook, Bloomington, IN) or a 0.073 Navien guide catheter (eV3, Irvine, CA) with a dual Y-adapter, so that the microcatheter and balloon can be introduced separately. However, they have also performed the procedure by puncturing both groins and introducing 2 separate guide catheters. With this technique a 5F and 6F guide catheter are usually introduced into the target vessel.
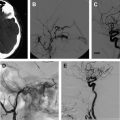
Under roadmap, the balloon first is advanced across the neck of the aneurysm. The microcatheter is then advanced into the aneurysm. The balloon is inflated across the neck of the aneurysm, causing temporary occlusion of the neck and parent vessel. The first coil is positioned within the aneurysm sac. The balloon is deflated to test the stability of the coil within the aneurysm sac. If no movement of the coil is observed, the balloon is reinflated and the coil detached. The coil is not detached if coil movement (meaning that the coil is not well anchored in the sac) is detected after balloon deflation. An angiogram is then performed. This procedure is repeated multiple times until the aneurysm no longer fills with contrast or has a dense coil mass within the confines of its lumen ( Fig. 2 ).
The balloon acts as a temporary wall across the neck of the aneurysm, allowing coils to be deflected off the balloon back into the aneurysm sac during placement. The choice of the first coil is the most crucial decision in being able to treat a wide-necked aneurysm. The coil diameter should be large enough to fully oppose the aneurysm wall and cross the neck well, thus providing friction between the coil and wall and limiting migration of the coil outside of the aneurysm. The coil diameter should be the largest that will form within the aneurysm sac. The coil length should also be the longest that will fit within the aneurysm sac. The large basket thus provided allows the coils to be strongly anchored within the aneurysm sac, and will also form a bridge across the neck to help prevent additional coils from migrating into the parent vessel. The balloon occlusion should not last more than 5 minutes. The size of the balloon depends on the volume of contrast and saline mixture introduced into the balloon; therefore, the balloon should only be inflated with the cadence syringe provided by the manufacture. Overinflation of the balloon may cause rupture of the parent vessel or aneurysm. The authors believe the balloon is best inflated with a 60:40 contrast/saline mixture, which provides good visibility of the balloon under roadmap and still leads to relative rapid deflation of the balloon. The balloon is tested on the table before introducing it into the patient. The balloon is also inflated in the lower cervical carotid or vertebral artery before introducing into the intracranial circulation, to determine if the balloon is working properly and to see if it can easily be seen under fluoroscopy. If the balloon will not deflate, the manufacturer recommends pulling negative pressure on the syringe connected to the Y-adapter. If all else fails, removing the wire from the balloon catheter will usually deflate the balloon; however, the entire system then has to be removed and prepped again before introducing it back into the body, because thrombus formation can occur within the balloon catheter, possibly making the balloon malfunction. Two other recently approved balloons that can be used for remodeling are the Ascent (Codman Neurovascular, Raynham, MA) and Scepter C Occlusion Balloon (Microvention-Terumo, Inc, Tustin, CA). The benefit of these two balloons is that they have a dual coaxial lumen, allowing for balloon inflation and coiling of the aneurysm with a single catheter. These balloons can also be advanced over a torquable .014 inch microwire.
Bifurcation wide-necked aneurysm
To treat a bifurcation aneurysm, the authors use a HyperForm balloon (Ev3), a very compliant low-pressure over-the-wire balloon that can conform to the vasculature being treated. Most interventionists believe this to be the balloon best suited for treating aneurysms located at arterial bifurcations or within small arteries. When the balloon is inflated, it may partially herniate into the aneurysm neck or the origin of the arterial branches emerging from the neck of the aneurysm. This configuration allows the parent vessel and arterial branches emerging from the neck of the aneurysm to remain patent with no compromise from the coils placed within the aneurysm sac.
Double-remodeling technique
Certain bifurcation aneurysms may not be able to be treated by a single-balloon remodeling technique. In these situations, another approach is to place two balloon catheters in a Y-configuration, one beside the other, both beginning proximally in the parent artery and ending distally within each of the branch vessels. The aneurysm is then coiled in a manner similar to that for the single-balloon remodeling technique. This technique allows the operator to treat certain aneurysms that would otherwise not be treatable by coil embolization.
A variation of the balloon remodeling technique is the conglomerate mass technique whereby multiple coils are placed into the aneurysm while the balloon is being inflated across the neck of the aneurysm for 5 to 10 minutes. Another variant is the balloon-in-stent technique for constructive endovascular treatment of ultra–wide-necked circumferential aneurysms, as reported by Fiorella and colleagues.
Results
In 2012, Pierot and colleagues published a review and concluded that the remodeling technique provides equivalent safety and better anatomic results in comparison with standard coiling, and can be widely used in the management of both ruptured and unruptured aneurysms. Spiotta and colleagues found no significant relationship between balloon inflation practices and ischemic events in a group of 147 patients undergoing balloon remodeling. Diabetic and older patients were more likely to have ischemic events develop. In 2008, Shapiro and colleagues published a literature review with a meta-analysis of the safety and efficacy of adjunctive balloon remodeling during endovascular treatment of intracranial aneurysms. These investigators concluded that there was no higher incidence of thromboembolic events or iatrogenic rupture with the use of adjunctive balloon remodeling in comparison with unassisted coiling, and also commented that balloon remodeling appears to result in higher initial and follow-up aneurysm occlusion rates. Forty wide-necked aneurysms were successfully treated with the HyperForm balloon remodeling technique by Mu and colleagues in 2008, with only 2 failed cases. Final results consisted of total occlusion in 34 cases (80.9%), subtotal occlusion in 4 (9.5%), and incomplete occlusion in 2 (4.8%). Of 22 patients treated in 2001 by Nelson and Levy, aneurysm occlusion was found in 17 of 20 patients on follow-up angiography at a mean of 19 months, the other 2 patients having died before follow-up. Layton and colleagues treated 73 of 221 aneurysms with balloon-assisted coiling over a 3-year period, and found no increased risk in thromboembolic complications in comparison with simple coiling techniques. In conclusion, there appears to be no increased risk of complications with the balloon remodeling technique. Aneurysm occlusion rates may be higher than with standard endovascular techniques, although there are few studies reporting the results of this form of treatment.
Stent-Assisted Coiling
Stent characteristics
Certain wide-necked and dysplastic aneurysms may not be treated with simple coiling or balloon remodeling; however, with the current availability of stents made to navigate the intracranial circulation, treatment from an endovascular approach is now possible. The disadvantages are that a permanent implant is placed into the artery, there is a lack of data on long-term patency, and the patient is subjected to the risk associated with the long-term use of antiplatelet medication. The stent acts as a scaffold that prevents the coils placed into the aneurysm sac from herniation into the parent vessel. The stent may also reduce the inflow into the aneurysm, promoting stasis and thrombosis of the aneurysm. In addition, the struts of the stent may provide a matrix for the growth of endothelial cells across the neck of the aneurysm.
A standard cerebral angiogram is obtained along with a 3D angiogram of the vessel in question. The dimension of the artery above and below the aneurysm is measured along with the size of the aneurysm and length of the neck. The targeted landing zone of the stent is determined. The self-expandable stent is sized to a nominal diameter 0.5 to 1.0 mm greater than the parent vessel at the targeted landing zone. The stent length should be centered on the aneurysm neck. The stent length is chosen to provide at least 5 mm of coverage proximal and distal to the aneurysm neck. If the parent vessel is tortuous, an attempt is made to place the distal and proximal aspect of the stent within a straight segment of the parent vessel.
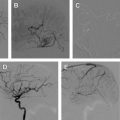
Initially, balloon-expandable coronary stents were used; however, these were difficult to navigate into the intracranial circulation. Inflating the balloon also subjected the patient to possible rupture of the parent vessel, which is not possible with a self- expanding stent. Three self-expandable intracranial stents are currently available on the market: Neuroform (Stryker), Leo (Balt Extrusion, Montmorency, France), and Enterprise (Codman Neurovascular, Raynham, MA). The Neuroform and Enterprise are only available in the United States. The major differences between these stents are the delivery system and whether they are closed-cell or open-cell. The Neuroform is an open-cell stent, whereas the Enterprise and Leo are closed-cell stents.
The self-expandable stent that comes preloaded into the delivery microcatheter is the Neuroform. The advantage is that the microwire remains within the lumen of the stent after its deployment, which may allow easier delivery of overlapping stents and catheterization of the aneurysm. The disadvantage is that navigating this system through the intracranial circulation can be difficult, owing to the poor torquability of the microwire because it is in contact with the stent. The system is also less flexible because the stent is loaded into the microcatheter during navigation. To overcome these problems, many operators will navigate a standard microcatheter and microwire into the distal intracranial circulation past the neck of the aneurysm. The wire is then exchanged for a 300-cm exchange-length microwire. This step is crucial, as during the exchange the distal tip of the microwire may produce a perforation of the distal vessel. The delivery microcatheter carrying the stent is then brought up over the exchange-length microwire, and the stent deployed. This step in the procedure is also crucial in that the distal tip of the microwire should also be observed to prevent perforation. Because of these difficulties the manufacturer has changed the platform for the delivery method so that it is similar to the methods of deployment of the Leo and Enterprise stent Fig. 3 .