Vascular malformations are congenital lesions secondary to errors in the development of arteries, capillaries, veins, or lymphatics. Most of these lesions are sporadic; however, a certain percentage present with syndromes. This article discusses the clinical features, natural history, and epidemiology of these lesions, and the diagnostic imaging features of vascular anomalies of the head and neck are presented. The percutaneous/endovascular treatment of each of the vascular anomalies is described, and surgical and additional treatment options are discussed briefly. The clinical outcomes of the main forms of treatment and level of evidence are presented.
Key points
- •
Venous malformations can be treated percutaneously by injecting a sclerosing agent. The most commonly used sclerosing agents include alcohol, sodium tetradecol, Ethibloc, polidocanol, and bleomycin.
- •
Multiple sclerosing agents have been used effectively for the treatment of lymphatic malformations, including absolute alcohol, sodium tetradecyl sulfate, doxycycline, Ethibloc, OK 432, and bleomycin.
- •
Approximately 8% of capillary malformations (CMs) are associated with Sturge-Weber syndrome and unilateral glaucoma. CMs are also associated with Klippel-Trenaunay, Parkes-Weber, macrocephaly CM, and CM-arteriovenous malformation (AVM) syndromes.
- •
Treatment of AVMs is difficult. Endovascular embolization is often the first treatment option with or without surgical resection. The main goal of any form of treatment is to eradicate the nidus.
- •
Endovascular treatment of an AVM can be performed from a transarterial approach, direct percutaneous puncture of the nidus, or a retrograde transvenous approach with alcohol, N -butyl-2-cyanoacrylate (n-BCA), or Onyx.
- •
Congenital hemangioma is a vascular lesion that completes the proliferative phase before birth. Two forms exist: the rapidly involuting congenital hemangioma (RICH) and the noninvoluting congenital hemangioma (NICH). RICHs involute more rapidly than hemangiomas of infancy, usually within the first 14 months of life. NICHs do not involute but grow in proportion to the child.
Introduction
In 1982, John Mulliken and Julie Glowacki proposed a classification of vascular anomalies based on clinical behavior, histology, and histochemistry. The International Society for the Study of Vascular Anomalies (ISSVA) accepted this classification in 1992. The classification divides vascular anomalies into 2 groups: tumors (eg, hemangiomas), the cause of which is endothelial cell proliferation, and vascular malformations, in which developmental error has resulted in abnormally formed vascular channels. In the tumor group, this article focuses on hemangiomas; the other tumor subtypes are beyond the scope of this review. Vascular malformations can be further subdivided into lesions consisting of arterial, capillary, lymphatic, venous, and fistulous networks. Furthermore, they can be further subdivided functionally based on the flow characteristics (ie, high-flow vs low-flow lesions). This article discusses the clinical features, natural history and epidemiology, and presents the diagnostic imaging features of vascular anomalies of the head and neck. The percutaneous/endovascular treatment of each of the vascular anomalies are presented. Additional treatment options, such as surgery are discussed briefly. Finally, the clinical outcomes of the main forms of treatment and level of evidence are presented.
Introduction
In 1982, John Mulliken and Julie Glowacki proposed a classification of vascular anomalies based on clinical behavior, histology, and histochemistry. The International Society for the Study of Vascular Anomalies (ISSVA) accepted this classification in 1992. The classification divides vascular anomalies into 2 groups: tumors (eg, hemangiomas), the cause of which is endothelial cell proliferation, and vascular malformations, in which developmental error has resulted in abnormally formed vascular channels. In the tumor group, this article focuses on hemangiomas; the other tumor subtypes are beyond the scope of this review. Vascular malformations can be further subdivided into lesions consisting of arterial, capillary, lymphatic, venous, and fistulous networks. Furthermore, they can be further subdivided functionally based on the flow characteristics (ie, high-flow vs low-flow lesions). This article discusses the clinical features, natural history and epidemiology, and presents the diagnostic imaging features of vascular anomalies of the head and neck. The percutaneous/endovascular treatment of each of the vascular anomalies are presented. Additional treatment options, such as surgery are discussed briefly. Finally, the clinical outcomes of the main forms of treatment and level of evidence are presented.
Venous malformations
Clinical Features
Venous malformations (VMs) can occur anywhere in the body, but are most frequently located in the head and neck. They can be solitary, small, well circumscribed, large, superficial, or infiltrative involving multiple tissue planes. The lesion is nonpulsatile, may have a light blue to deep purple color, and can be associated with telangiectasias, varicosities, or ecchymosis. The mass may increase in size in a dependent position, with a tourniquet, or during a Valsalva maneuver. Superficial lesions are soft and compressible and can usually be emptied of blood. Patients usually present with pain associated with compression on the surrounding nerves or thrombosis of a portion of the mass. An increased level of D-dimer has been determined to be highly specific for a VM and can help to distinguish VMs from lymphatic malformations and slow-flow Klippel-Trenaunay syndrome from high-flow Parks-Weber syndrome.
VMs are usually isolated findings; however, they may be associated with the following syndromes ( Table 1 ):
Klippel-Trenaunay syndrome
Blue rubber bleb nevus (BRBN) syndrome
Mucocutaneous familial VMs
Glomuvenous malformation
Maffucci syndrome
Proteus syndrome
Bannayan-Riley-Ruvalcaba syndrome
CLOVES/S syndrome
| Syndromes | Vascular Tumors | Vascular Malformations | |||
|---|---|---|---|---|---|
| Infantile Hemangioma | Venous | Capillary | Lymphatic | Arterial or Arteriovenous | |
| PHACES | ● | ||||
| LUMBAR | ● | ● | |||
| Kasabach-Merritt | ● | ||||
| Maffucci | ● | ● | |||
| Klippel-Trenaunay | ● | ● | ● | ● | ● |
| Gorham-Stout | ● | ● | ● | ||
| Proteus | ● | ● | ● | ||
| Parkers-Weber | ● | ● | ● | ||
| Sturge-Weber | ● | ● | ● | ||
| CM-AVM | ● | ● | |||
| Familial cerebral VM | ● | ||||
| Familial cutaneomucosal VM | ● | ||||
| Blue rubber bleb nevus | ● | ||||
| Bockenheimer | ● | ||||
| Cobb | ● | ● | |||
| Osler-Weber-Rendu | ● | ● | ● | ||
| Beckwith-Wiedemann | ● | ● | |||
| Louis-Barr | ● | ||||
| Down | ● | ||||
| Turner | ● | ||||
| Wyburn-Mason | ● | ||||
| Dandy-Walker | ● | ||||
| Von Hippel-Lindau | ● | ||||
Natural History/Epidemiology
VMs are present at birth. They are not always clinically apparent and tend to grow in proportion to the growth of the child. The growth is most pronounced during puberty and pregnancy. These are congenital lesions that affect boys and girls with equal frequency with a reported incidence of 1 to 2 per 100,000 births and a prevalence of 1%.
Most VMs (95%) are sporadic, but can be seen in several heritable conditions. The molecular basis for sporadic occurrence has yet to be discovered, however there are familial cases where the genetic defect has been localized on a specific chromosome. In 1994/1995, 2 families were identified to have autosomal dominant inherited cutaneous and mucosal VMs. Genetic analysis mapped a locus for both of these families to chromosome 9p21. These families shared a mutation resulting in an arginine to tryptophan substitution R849W in the gene that encodes for the kinase domain of the endothelial cell receptor Tie2. In 1999, 4 more families with autosomal dominant inherited VMs were identified. Only 1 of those families shared the same mutation as the previous 2 reports. The second family had a novel hyperphosphorylating Y897S mutation in the TIE2 gene. The other families showed no evidence of linkage to 9p21, which suggests genetic heterogeneity. Multifocal VMs are most commonly seen in the familial forms of VM, including the following: BRBN syndrome, mucocutaneous familial VMs, glomuvenous malformation, and Maffucci syndrome.
Diagnostic Imaging
Ultrasonography
On grayscale imaging, VMs are usually hypoechoic to anechoic with the shape of tubular structures. Some lesions can have a heterogeneous echotexture if phleboliths or different forms of thrombus are present within the lesion. Doppler flow is usually a monophasic low-velocity flow. Sometimes flow can only be seen with compression and release of the lesion.
Computed tomography
On noncontrast computed tomography (CT), VMs are usually hypoattentuating, however they can be heterogeneous depending on the amount of fatty tissue within the lesion. Phleboliths or dystrophic calcifications can be seen within the lesion. After the administration of contrast, the lesion usually enhances on the periphery and then fills in centrally on the delay images. CT is excellent at looking for boney involvement from the lesion. Magnetic resonance (MR) imaging is better at characterizing the relationship of the lesion with surrounding soft tissue structures.
MR imaging
VMs usually appear as hypointense to isointense on T1-weighted imaging. They can, however, have areas of bright signal on T1-weighted images if the lesion has fat, subacute blood products, or certain types of calcifications contained within the lesion. On T2-weighted images, VMs have high signal intensity. Gradient echo sequences usually show areas of low signal corresponding to calcification, hemosiderin, or thrombus. The best sequence to determine the full extent of the lesion and its relationship with surrounding soft tissue structures is the T2-weighted sequence. On T1-weighted postcontrast imaging, the lesions usually demonstrate early peripheral enhancement that later fills in centrally on delayed imaging.
Diagnostic venography
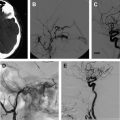
Contrast venography is helpful in the anatomic characterization of a VM. It is performed to determine the full extent of the malformation and its draining veins. It is also helpful in confirming patency of the deep venous system in the extremity. Venography is also performed to determine the volume of contrast needed to fill the malformation before it empties into normal draining veins.
Based on the pattern of venous drainage, VMs can be divided into 4 types. This drainage pattern is a reflection of the response to treatment and rates of complications. Types I and II respond best to sclerotherapy with a better control rate and fewer sessions to achieve control. Types III and IV have a higher rate of complications.
Type I: isolated malformation without discernible venous drainage
Type II: lesion draining into normal veins
Type III: lesion draining into dysplastic veins
Type IV: lesion consists primarily of venous ectasia
Treatment
VMs can be treated percutaneously by injecting a sclerosing agent. The most commonly described sclerosing agents ( Box 1 ) include alcohol, sodium tetradecol, Ethibloc, polidocanol, and bleomycin. The procedure is performed under general anesthesia because injection of the sclerosing agent can be extremely painful. A Foley catheter is placed in the bladder to monitor urine output and color because some patients may develop hemoglobinuria. This is most likely to happen in younger patients and in patients with large lesions. In addition, if an extremity is being treated, we start an intravenous (IV) line distal to the lesion to infuse saline and heparin overnight to flush out the sclerosing agent and prevent acute deep venous thrombosis. Sclerotherapy is performed by cannulating the lesion percutaneously under ultrasonography or fluoroscopy ( Fig. 1 ). Contrast material is injected to define the extent of the lesion, determine the flow rate within the lesion, the communication with normal veins, and the presence of a possible arterial component, and to give an estimate of the volume of sclerosing agent needed to fill the lesion. If a large draining vein is visualized, this can be treated with embolization using coils, liquid embolic agents, or with temporary balloon occlusion to increase the contact time of the sclerosing agent with the VM and to prevent egress of the agent into the systemic circulation. This may also be performed with a tourniquet, blood pressure cuff, or manual compression. The sclerosant is then injected under fluoroscopic guidance to decrease the risk of extravasation and overfilling of the lesion, and to limit undesired egress of the sclerosing agent into the normal deep veins. Overfilling of the lesion may also be prevented by the 2-needle access technique, which allows decompression of the lesion through the second needle access. Cone beam CT can be performed to evaluate the anatomic distribution of the sclerosant administered and compare the distribution with the preprocedure imaging. After the needles are removed, a 20 to 30 mm Hg compression stocking is placed over the area of concern so that the veins do not refill with blood. Compression allows maximum contact of the damaged endothelium of the vein wall, promoting fibrosis and scarring. The procedure is then repeated at intervals of 8 to 10 weeks until there is no recanalization, swelling, or pain from the lesion. Diffuse VMs are much more difficult to treat. The area of maximum symptomatology is usually targeted because these lesions cannot be completely obliterated.
- •
Alcohol
- •
Sodium tetradecol
- •
Alcoholic solution of zein (Ethibloc)
- •
Polidocanol
- •
Bleomycin
- •
OK-432 (picibanil)
- •
Doxycycline
After sclerotherapy the lesion should feel firm to palpation. Swelling is generally maximum 24 hours after the procedure. We recommend placing a custom-fitted class II compression garment over the lesion as soon as possible. If the lesion involves the airway, the patient may need to be kept intubated overnight in the intensive care unit. The patient is given IV steroids in the hospital and then sent home on a tapered dose pack. The affected area is kept elevated above the heart with ice packs applied to minimize swelling. Patients receive IV fluids at twice the maintenance dose before, during, and after the sclerotherapy for 24 hours. Urine output is closely monitored for hemoglobinuria. Appropriate IV and oral analgesics and antiinflammatory agents are prescribed in the hospital and for 7 days after discharge.
Patients with extensive limb lesions should be instructed from childhood in the proper use of compression garments. Compression helps to decrease the discomfort associated with the lesion, protects the overlying skin, limits swelling, and improves localized intravascular coagulation.
In patients with extensive VMs in whom a low fibrinogen level is present, the use of low-molecular-weight heparin is recommended for 2 weeks pretreatment and possible cryoprecipitate transfusion if still low on the day of the procedure. This therapy reduces the consumptive coagulopathy of the extensive VM and potentially lowers the recanalization rate.
Sclerosant drugs
Absolute ethanol (95%–98%) is probably the most common agent used for sclerotherapy. It is the most effective sclerosant agent available, however it is also the most toxic. Ethanol works by causing instant precipitation of endothelial cell proteins and rapid thrombosis. It may also cause transmural vessel necrosis resulting in diffusion into the surrounding tissues. Serious side effects from ethanol injection include massive swelling, tissue necrosis, hypoglycemia, peripheral nerve injury, central nervous system depression, hemolysis, pulmonary vasospasm, cardiac arrhythmias, and electromechanical disassociation. Given the serious side effects, patients must be closely monitored during procedures involving ethanol. Some practitioners even suggest monitoring of pulmonary artery pressure during and for a short period after the procedure. Ethanol blood levels correlate directly with the amount of ethanol injected. Mason and colleagues demonstrated that a level of more than 1 mL/kg may put patients at increased risk of respiratory depression, cardiac arrhythmias, seizures, and rhabdomyolysis. A total dose of 1 mL/kg (or 60 mL) per session should never be exceeded. In pediatric patients, a maximum dose of 0.5 mg/kg per session is recommended. Ethanol can be injected in an undiluted form; however we believe this is dangerous because the agent cannot be identified under fluoroscopy. We usually dilute alcohol with water-soluble liquid or oily contrast medium during injection. Alcohol should not be mixed with Visipaque because this causes precipitation of the contrast medium. In a series of 60 patients with VM involving the head and neck treated with ethanol, Su and colleagues reported 68% complete response (>90% volume reduction), 25% marked response (>50% volume reduction), and 7% moderate response (<50% volume decrease) with a 10% complication rate (level IV evidence). In a series of 158 patients with VM, 16% of patients had a good response defined by clinical examination and a decrease in size of 30% or greater on MR imaging; unfortunately 27% of these patients experienced a complication (level IV evidence). In another series of 87 patients with craniofacial VMs treated with ethanol sclerotherapy, 32% had an excellent response (>75% volume reduction), 52% a good response (>25% volume reduction), and 16% a poor response (25% volume decrease) with a 5% complication rate (level IV evidence).
Detergents
Detergents used for sclerotherapy of VMs include sodium tetradecyl sulfate, polidocanol, sodium morrhuate, and ethanolamine. Sodium tetradecyl sulfate, is approved in the United States for treatment of varicose veins. Polidocanol has US Food and Drug Administration approval for sclerosis of spider and reticular veins. Similar to ethanol, all of the drugs damage the endothelial cells, resulting in thrombosis and fibrosis. Detergents may be mixed with water-soluble or oily contrast medium before injection. These agents are believed to have a low rate of complications compared with ethanol, but a greater tendency toward recanalization. Reported complications include cardiovascular collapse, skin pigmentation, skin necrosis, anaphylaxis, and hemoglobinuria. Foaming the sclerosant by mixing the drug with air has also become popular. The theory is that the foam probably results in better contact between the drug and vein wall along with prolonged displacement of the blood with the lesion. Foam is made easily by mixing 10 mL of sclerosant with 3 mL of Ethiodol and 5 to 10 mL of air through a 3-way stopcock. Patients receiving large volumes of detergent sclerosants may need aggressive hydration and alkalization of the urine to treat hemoglobinuria. In a prospective randomized controlled trial comparing foamed versus liquid polidocanol or ethanolamine in 89 patients with VMs, 90% of patients had partial or complete response in the foamed group versus 63% in the liquid group ( P = .002) (level I evidence). In another series of 50 patients with VMs treated with polidocanol foam, complete resolution occurred in 38% of patients, a reduction in size of 50% or more in 30% of patients, a reduction in size of between 0% and 50% in 26% of patients, and no change in 8% of patients (level IV evidence). Reported complications were noted in 8% of the patients. Furthermore, in a series involving 26 patients with VM, use of ethanolamine oleate showed 49% excellent, 39% good, 12% fair, and 0% poor results based on clinical examination (level IV evidence).
Bleomycin
Bleomycin, an antibiotic and antitumor agent originally derived from Streptomyces verticillus in 1966, causes an inflammatory response in endothelial cells. Bleomycin can be mixed with water-soluble liquid or oily contrast medium. There is some concern about the use of this agent because of the association with pulmonary fibrosis in the setting of chemotherapy. The threshold dose for pulmonary fibrosis and interstitial fibrosis is 450 mg; doses for sclerotherapy are typically 0.5 to 1 mg/kg for pediatric patients and 1 to 15 mg in adults. Reported complications include skin ulceration, skin pigmentation, laryngeal edema, flulike symptoms, cellulitis, nausea and vomiting, and focal alopecia. In a series of 31 patients with VM treated with percutaneous injection of bleomycin, complete resolution occurred in 0%, marked decrease (≥50%) in size in 34%, minimal decrease (≤50%) in size in 31%, stable size in 34%, and increase in size in 0% on postprocedure MR imaging (level IV evidence). Complications were seen in 12.5% of patients and consisted predominantly of transient skin pigmentation and cellulitis. In another series of 32 patients treated with percutaneous bleomycin sclerotherapy, complete resolution of the lesion was found in 32% of patients and significant improvement in 52% of patients by clinical examination (level IV evidence).
Liquid embolic agents
BCA has a limited role in the treatment of vascular malformations because of its high cost, limited reabsorption, and the formation of a hard mass after injection that may last for many months. Burrows and colleagues suggest this form of treatment for preoperative embolization before surgical resection. BCA is most commonly used in the treatment of intra-articular VMs because injection of a sclerosing agent could cause damage to the articular cartilage. This form of treatment has also been described for an orbital VM (level IV evidence). Ethylene vinyl alcohol copolymer (Onyx), a newer liquid polymer, may also be useful for preoperative embolization of VMs before resection.
Other forms of treatment
Endovascular diode laser therapy has been reported in a small series of VMs with a good response rate at 14-month follow-up (level IV evidence). In patients with Klippel-Trenaunay syndrome, endovenous laser ablation of the marginal vein may be a useful alternative therapy. Percutaneous, interstitial (nonendovascular) laser photocoagulation of VM using the diode or neodymium:yttrium-aluminum-garnet (Nd:YAG) lasers via a fiber-optic delivery system has also been reported with reasonable success (level IV evidence). Because of the controlled delivery of energy, and with external cooling, this modality may be useful for extensive superficial cutaneous/subcutaneous lesions or isolated anatomic locations such as the digit or tongue where sclerosis or embolization may carry a higher risk of surrounding tissue necrosis.
Surgical resection of VMs is indicated only when complete resection without a resulting functional or anatomic deficit is possible. Surgery plays a limited role in the management of extremity lesions, but if performed, localized coagulopathy must be controlled before surgery. Surgery may also help to avoid joint distention from repeat hemarthrosis if joint involvement with a VM of the synovium is present.
Lymphatic malformation
Clinical Features
Lymphatic malformations (LMs) are present at birth and are composed of abnormal dilated lakes of lymphatic tissue that result from defective embryologic development of the primordial lymphatic channels. LMs can be classified radiographically as macrocystic (cysts ≥2 cm), microcystic (cysts <2 cm) or mixed, which has important implications for treatment. Macrocystic lesions are most commonly located in the neck, axilla, and chest wall, and, when large, may interfere with the birth process. Microcystic lesions usually present as diffuse soft tissue thickening, often associated with vesicles of the skin and mucosa with an overlying capillary malformation. The lesions grow in proportion to the patient. They may undergo periodic swelling often associated with signs of inflammation, which may be spontaneous or related to a regional infection. Acute swelling of the lesion may also be related to hemorrhage or lymphatic obstruction. Symptoms caused by LMs are typically related to the localized mass effect on adjacent structures. On physical examination, LMs are mobile and have a boggy or cystic consistency. The lesions cannot be decompressed and do not distend with the Valsalva maneuver like VMs. The presence of lymphatic endothelium can be confirmed by staining with D2-40 antibody.
Infection is usually common in suprahyoid LMs with mucosal involvement. Patients with airway involvement can present with stridor and sleep apnea. In one series, tracheostomy placement occurred in 5% of patients with airway compromise at birth. Orbit LMs can present with pain, swelling, proptosis, blepharoptosis, and emblyopia. An underlying cerebral developmental anomaly is seen in 45% of patients with orbital involvement.
Natural History/Epidemiology
Developmental defects during embryonic lymphangiogenesis result in LMs. They tend to grow in proportion with the growth of the child. The growth is most pronounced during puberty and pregnancy. The incidence is reported at between 0.02% and 0.05%, and represents approximately 3 of 100,000 hospital admissions. More than 50% of LMs are identified at birth, with 80% to 90% usually identified by 2 years of age. Approximately 75% of the lesions occur in the head and neck region. Spontaneous regression is rare and has been report in less than 4% of cases. Multiple genes have been described in the process of lymph angiogenesis including VEGFR3, VEGFC, Ang2, Lyve1, Nrp2, and podoplanin. No evidence exists for heritable LM. Cervical LMs do occur in association with Klippel-Trenaunay, Turner, and Noonan syndromes, as well as trisomy 13 and 18.
Diagnostic Imaging
Ultrasonography
Macrocystic lesions appear on grayscale imaging as multiple anechoic or hypoechoic cystic spaces with internal septations and debri. Microcystic lesions appear as an ill-defined hyperechoic mass on grayscale imaging.
CT
LMs appear as fluid-filled low-attenuation lesions, with occasional fluid/fluid levels that represent hemorrhage into the cystic structure. Peripheral enhancement of the walls may occur, however there is no central filling of the lesion on delayed images like a VM. The internal septations are commonly not seen.
MR imaging
Macrocystic LMs appear as fluid-filled lesions with a single locule or multiple loculations, displaying high signal on T2-weighted images and low signal on T1-weighted images. Occasionally, a fluid/fluid level can be seen in the setting of internal hemorrhage. Postgadolinium T1-weighted images show minimal or absent septal enhancement. Microcystic lesions demonstrate intermediate signal intensity on magnetic resonance imaging (MRI) T1 and T2 spin echo sequences.
Treatment
Multiple sclerosing agents have been used effectively for the treatment of LMs, including absolute alcohol, sodium tetradecyl sulfate, doxycycline, Ethibloc, OK 432, and bleomycin.
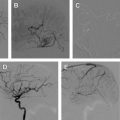
The technique for sclerotherapy of LMs consists of cannulation of each cyst with a standard angiocatheter under ultrasonographic guidance. As much fluid as possible is aspirated from the cyst. Contrast is injected under fluoroscopic guidance to identify the size of the lesion. The sclerosing agent is mixed with a contrast medium and the cyst injected under fluoroscopic guidance ( Fig. 2 ). Smaller macrocystic lesions can be treated with aspiration and sclerotherapy without catheter drainage. For large lesions, a pigtail catheter may be placed into the cyst and the lesion drained and injected over several days. Microcystic lesions are a challenge to treat and generally require multiple injections. Treatment is continued until imaging identifies no treatable cysts or the patient is satisfied with the clinical outcome.
Doxycycline
Doxycycline is generally instilled into the cyst as a solution of 10 mg/mL. The dose of doxycycline can range from 150 mg to 1000 mg depending on the size of the lesion. In neonates, the maximum recommend dose at 1 time is 150 mg, because higher doses may cause hemolytic anemia, hypoglycemia, and metabolic acidosis. Blood glucose is monitored 2 hours after the procedure in neonates. If the patient develops hypoglycemia, IV dextrose can be administered. A few small series have described a 93% to 100% complete radiographic response for treatment of macrocystic LMs (type IV evidence). Lower response rates are noted for microcystic LMs.
Detergents
There is limited literature on the used of detergents as sclerosant agents for LMs. One study described the use of 98% ethanol and 3% sodium tetradecyl sulfate, with a 100% complete radiographic response rate (level IV evidence). A second series described the use of foamed sodium tetradecyl sulfate, for the treatment of 6 intraorbital LMs with a reduction in lesion size in all patients (level IV evidence).
OK-432 (picibanil)
OK-432 (picibanil) is a lyophilized mixture of group A Streptococcus pyogenes initially developed as an immunotherapeutic agent in the treatment of gastric and lung carcinoma with the first use as a sclerosant in the treatment of an LM described in a case report in 1987 (level IV evidence). OK-432 is not approved in the United States. Direct injection of the solution into an LM has been shown to increase intralesional cytokine levels, namely tumor necrosis factor, interleukin 6 (IL-6), IL-8, interferon-α, and vascular endothelial growth factor in patients with a response to treatment with OK-432. A review of the literature by Poldervaart and colleagues showed an 88% excellent response rate for macrocystic lesions. Microcystic lesions demonstrated an excellent response rate in 27%, good in 33%, and poor in 40% of cases. Adverse side effects were mild and consisted of fever, lethargy, and local inflammation. A phase II trial was conducted in the United States on 182 patients with LMs. In this trial, 94% of the patients with macrocystic disease had a response to treatment, 63% of the patients with mixed macrocystic/microcystic disease had a response to treatment, and 0% of the patients with microcystic disease responded to treatment (level I evidence). In 2 other series, complete response rates of 76% and 83.5% were noted (level IV evidence).
Alcohol solution of zein
Alcoholic solution of zein (Ethibloc; Ethicon, Norderstedt, Germany) is a biodegradable thrombogenic solution consisting of a combination of zein (corn protein), sodium diaatrizoate, and oleum in ethanol. It has been used in Canada and Europe but is currently not approved in the United States. Dubois and colleagues reported a more than 95% volumetric regression by CT in 64% of lesions and more than 50% in the remainder of macrocystic and microcystic lesions (level IV evidence). Reported complications in this series included a minor local inflammatory reaction and an initial increase in size of the lesion in all patients. In another study of 65 patients with LMs treated with Ethibloc equal to 10% of the lesion volume, 49% achieved excellent results, 35% showed satisfactory results, and 16% had poor results (level IV evidence). Complications included a localized inflammatory reaction and self-limiting external leakage of the Ethiobloc at a mean of 2 months after therapy.
Bleomycin
Bleomycin is also used for sclerotherapy of LMs. The previous discussion on the use of bleomycin for VMs also holds true for LMs. In a series of 70 patients with LM treated with intralesional injection of bleomycin, 47% of patients achieved excellent results, 36% achieved good results, and 17% had poor results. Seven percent of the group had recurrence (level IV evidence). Mild side effects consisted of fever, pain, swelling, and leukopenia. In another series of 200 patients with LM treated with intralesional injection of bleomycin-A5, 87% of patients demonstrated complete lesion resolution with a recurrence rate of 13% (level IV evidence). Complications were mild; anorexia and fever were the most common complications reported.
Laser therapy
Laser therapy is generally reserved for lesions with superficial cutaneous/mucosal vesicles. Microcystic lesions of the oral cavity and tongue have also been treated with the carbon dioxide laser and the Nd:YAG with success (level IV evidence).
Radiofrequency ablation
In a series of 11 patients, Grimmer and colleagues reported a response rate of 62% with radiofrequency ablation in patients with microcystic lesions of the lips, tongue, buccal mucosa, and floor of the mouth (level IV evidence).
Surgery
Surgery is usually performed after an attempt at sclerotherapy. Depending on the location of the lesion, removal can be disfiguring with a high incidence of recurrence.
Capillary malformations
Clinical Presentation
Capillary malformations (CMs) are present at birth and grow commensurately with the child. CMs may be single or multiple, involve only the dermis, are well demarcated, and appear pink in infancy and may become dark purple with age. Symptoms related to sporadic CM are primarily cosmetic although they can develop severe bleeding after minor trauma. CMs can be associated with overgrowth of the affected limb or face with certain syndromes.
Natural History/Epidemiology
CM, also termed port-wine stain, capillary hemangioma, nevus flammeus, angel’s kiss, and stork’s bite, are common vascular malformations with an incidence of 0.3%. CM is usually sporadic, but families with a dominant inheritance pattern with incomplete penetrance have been reported. CM-AVM syndrome has been linked to chromosome 5q14-21, with a defect in the RASA1 gene that encodes a p120 RasGTPase-activating protein, which is involved in cell adhesion and angiogenesis. Most CMs in the head and neck occur in the V1 and V2 distribution of the trigeminal nerve. Approximately 8% of CMs are associated with Sturge-Weber syndrome and unilateral glaucoma. CMs are also associated with Klippel-Trenaunay, Parkes-Weber, macrocephaly CM, and CM-AVM syndromes.
Diagnostic Imaging
There is little role for imaging except to exclude more serious disorders associated with CMs such as in Sturge-Weber syndrome. Ultrasonography may reveal areas of hyperechogenicity on grayscale images with minimal flow on Doppler imaging. Given the superficial location of these lesions, a high-frequency ultrasound probe (>10 MHz) is necessary to fully characterize the lesion. CT imaging findings are usually minimal even with extensive disease. On MR imaging, the skin may appear thickened with abnormal signal in the superficial soft tissue. The lesion may enhance after IV contrast administration. The value of CT and MR imaging for diagnosing CM is in ruling out more serious syndromes.
Treatment
Photocoagulation with pulsed dye laser (PDL) has been shown to be effective in improving the appearance of CMs (level IV evidence). However, controversy exists on whether PDL provides long-term benefit (level IV evidence).
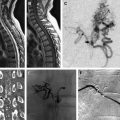
There is no indication for embolization of these lesions, except in reconstruction of a hyperemic facial skeleton. Preoperative particulate embolization of the lesion may decrease blood loss during surgical reconstruction ( Fig. 3 ).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree