Key Points
- •
When considering ultrasound machine purchasing, the scope of examinations, clinical setting, and skill set of the end users should guide the choice of make and model, transducers, and software packages.
- •
A structured quality assurance process is an essential component of any clinical point-of-care ultrasound program.
- •
Appropriately acquired and interpreted point-of-care ultrasound exams are eligible for billing when the required supporting documentation is included.
Background
Point-of-care ultrasound has expanded rapidly over the past decade. In 2006, the United States ultrasound sales market hit a record high of 1.33 billion dollars. Although 60% of these sales represented expenditures in traditional imaging specialties, such as radiology, 40% of the total sales were due to increased demand for compact, hand-carried ultrasound machines designed specifically for point-of-care applications. With the increasing demand, manufacturers have responded by creating unique devices with a broad range of options and features. It is important to consider the skill level and needs of the providers when selecting a manufacturer, make, and model.
Ultrasound Equipment
Size
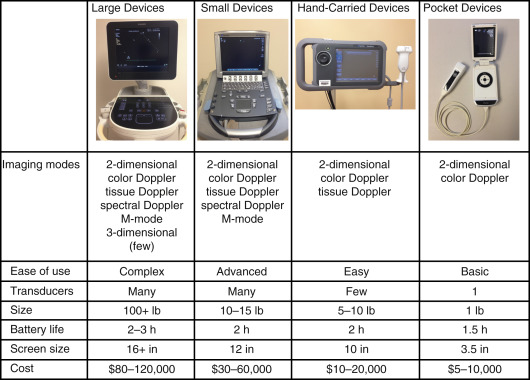
When choosing a point-of-care ultrasound machine, size is an important consideration. In general, smaller ultrasound machines have less functionality (e.g., Doppler may not be an available feature), but smaller machines are more portable and usually less expensive. Thus, one must balance portability, functionality, and cost ( Table 44.1 ).
In certain clinical settings, such as an intensive care unit or emergency department, it may be important to buy a compact machine, or even a handheld device, that can be rapidly moved to the bedside of critically ill patients. For other clinical settings, such as an outpatient clinic, where the machine is stationed in one examination room, a larger, less portable ultrasound machine with increased functionality may be a better choice. With most portable ultrasound machines attached to a cart, the size of the cart is as important a consideration as the size of the machine itself.
Power Source
Battery power determines how long an ultrasound machine can be used without requiring charging, which affects portability. Ideally, battery life should allow for several hours of scanning, giving providers the freedom to perform several ultrasound examinations without being tethered by an electrical cord. The ultrasound machine may also serve as a power source for other devices through USB ports, such as a printer, video recorder, gel warmer, bar code scanner, wireless bridge, or other institution-specific devices. The start-up time of an ultrasound machine is an important consideration, especially when caring for acutely ill patients with potentially life-threatening conditions. A long start-up time will be a barrier to use, particularly when patient care decisions must be made emergently.
Transducer Selection
The clinical applications that are most likely to be performed determine what types of transducers are needed. Phased-array, linear array, curvilinear, and endocavitary transducers are the most common types of transducers. Transducers can be easily damaged because they have fragile internal construction containing piezoelectric elements, and damaged transducers often cannot be repaired. The approximate cost of replacing a typical transducer ranges from $6000 to $12,000. Selection of transducers that are durable to function in high-acuity, portable settings with multiple users is important. Many manufacturers offer service contracts to repair or replace damaged transducers, and it is important to ensure that your vendor is able to ship replacement transducers in a timely manner to minimize interruption of workflow.
Features and Functions
The buttons for adjusting gain and depth must be easily identified. Many machines have a default or auto setting for gain that resets the gain throughout the image to the default setting for the selected exam. In addition to a freeze button to capture still images, most machines have a cine-loop function. The cine-loop function stores images from several seconds and allows providers to scroll through and select the highest quality images. Other features, such as tissue harmonics, elastography, and spatial compounding, may be important, depending on the practice setting. A practical consideration is selecting an ultrasound machine with an ergonomic control panel. Trackballs are more precise and easier to use with a gloved hand when compared to trackpads, but they are also more susceptible to being damaged or clogged by ultrasound gel. Calculation packages that can be used when performing certain ultrasound exams, such as fetal ultrasonography, are included features with some software packages.
Maintenance
Some manufacturers include a service warranty with the purchase of an ultrasound machine, while others may offer purchase of a separate warranty. Service contracts often cost 10–15% of the total purchase price on an annual basis, which adds substantially to the cost of ownership; however, we recommend purchase of a comprehensive service warranty, particularly when multiple healthcare providers will be using the machine.
Transducers may serve as vectors of bacterial transmission and should be disinfected before and after each patient contact. Transducers should never be autoclaved or subjected to high heat, electricity, or pressure because the piezoelectric elements of transducers are very sensitive and easily damaged. Transducers that do not make contact with mucous membranes can be cleaned with nonabrasive soap followed by an intermediate-level disinfectant after each use. Transducers that make contact with mucous membranes, including endocavitary transducers, should be used with a disposable cover, and should be cleaned with nonabrasive soap and water, followed by a high-level disinfectant after each use. When imaging a grossly infected or bloody surface, a disposable transducer cover should be used followed by high-level disinfection. Consult institutional policies for local transducer disinfection requirements. Information about appropriate disinfectants can be obtained from the ultrasound manufacturer or the FDA website. Adherence to these guidelines will ensure transducer longevity and compliance with infection control practices.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree