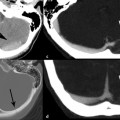
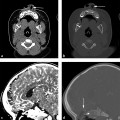
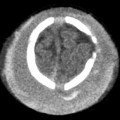
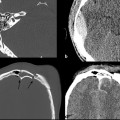
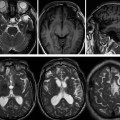
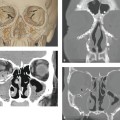
Evidence-Based Imaging and Prediction Rules: Who Should Get Imaging for Mild Traumatic Brain Injury? It is estimated that more than 1.3 million people are treated for mild traumatic brain injury (mTBI) in emergency departments (EDs) in the United States each year.1 Although most patients with mTBI can be sent home after a brief period of observation, a small proportion will show neurologic deterioration and may require hospitalization or, rarely, neurosurgical intervention. It has been reported that 10 to 15% of patients with mTBI whose Glasgow Coma Scale (GCS) scores of 15 will have acute abnormalities on noncontrast head computed tomography (CT), but only 1% of this group will have lesions that require intervention.1–5 Between 5 and 15% of mTBI patients will have persistent disability 1 year after the initial injury, including persistent headaches, cognitive impairments, and difficulties with complex tasks.6,7 Some patients with mTBI will not be able to return to routine activities and work for prolonged periods, taxing the U.S. economy an estimated 17 billion dollars yearly.8 In patients with clinically important brain injury, CT imaging can provide an efficient and accurate diagnostic tool such that neurosurgical intervention can prevent adverse outcomes from intracranial hemorrhage, cerebral herniation, or hydrocephalus. Overuse of CT in the evaluation of mTBI exposes the patient and the health care system to unnecessary costs, incurring $750 million in charges.9 More than one million head CT scans are performed in the United States in the setting of mTBI, most secondary to falls or motor-vehicle accidents, and approximately 90 to 95% of these examinations are negative.1,8–11 In recent years, there has been a fivefold increase in the use of head CT in the setting of mTBI, without an increase in the diagnosis of life-threatening conditions or the rates of hospital admissions.12,13 A part of imaging overuse in the setting of mTBI is related to medical legal concerns, exemplified by the fact that states that have passed tort reform laws have the lowest ED CT imaging use rates for mTBI. In addition to the health care costs, unnecessary head CT imposes ionizing radiation on the head, which is a serious concern, especially for pediatric trauma patients. Although the brain is not a radiosensitive organ and the radiation dose of a routine head CT is relatively low, approximately 2 millisieverts (similar to a chest radiograph), judicious use of CT scans is nevertheless essential. The cancer-related risk of ionizing radiation is age and gender specific, but it is estimated that there is one radiation-related cancer per 4360 to 14,680 head CT scans in adult patients, depending on age and sex.14 There is variability in the nomenclature used to describe mTBI, as well as the defining criteria and classification. Terms such as minor TBI, mild TBI, concussion, and low-risk TBI are used interchangeably. Most are classified as minimal or minor to mild; some define minimal injuries as having suffered no loss of consciousness or post-traumatic amnesia (LOC or PTA) and typically not requiring hospitalization. There are many definitions of mTBI, with minor variations, although most incorporate a history of LOC, amnesia, disorientation, or transient focal neurologic signs or seizure in the setting of blunt traumatic or acceleration/deceleration injury, with a GCS score of 13 to 15.8,11,15,16 Some have advocated placement of patients with GCS scores of 13 into the “moderate” TBI group because of the significant number of injuries requiring neurosurgical intervention17–19; however, in most classification schemes, these patients are still included in the mTBI category. The New Orleans Criteria (NOC) defines mTBI as loss of consciousness after trauma in a neurologically intact patient with GCS score of 15.2 Some have advocated inclusion of patients without LOC or PTA, considering that in this setting, if other risk factors for traumatic brain injury are present, intracranial abnormalities can be found at a similar rate to that in patients without LOC or PTA.20 The GCS was initially developed by Teasdale and Jeannette to reliably evaluate comatose patients with head injury and was meant to serve as a simple assessment tool by relatively inexperienced clinicians to serially and reliably evaluate comatose patients.21–23 The scale was not developed to evaluate mTBI, although it has proven useful in moderate and severe TBI assessment and to guide the need for neurosurgical intervention. There is growing sentiment that TBI classifications based on GCS scores are limited, with grouping of heterogeneous trauma patient populations with very different prognoses into broad categories. One prospective study found that despite having GCS scores of 13 to 15, patients with intracranial injuries on imaging performed similarly to patients with moderate TBI on neuropsychological testing.19 The Veterans Affairs and Department of Defense definition of mTBI includes normal structural imaging (i.e., head CT), thus classifying patients with imaging abnormality into moderate TBI. In the setting of mTBI, single-time-point GCS scores are of limited clinical value because they are not accurate for intracranial injury or prognosis. Rather, serial GCS score evaluation provides more prognostic information.6 The difficulty facing clinicians is determining the apparently well-appearing, neurologically intact patients who may have underlying intracranial injury that will require neurosurgical intervention. A secondary and more elusive goal is to identify patients with risk for postconcussive symptoms. There is considerable heterogeneity in opinions regarding indications for ordering CT heads in the setting of mTBI. Some advocate ordering CT head examinations for all patients with mTBI, regardless of clinical presentation,16,24,25 whereas others26–29 advocate more judicious use of CT. CT ordering practices have varied significantly between institutions, as well as between clinicians from the same institution. The development of a clinical decision-making tool, using clinical history and physical examination, to identify blunt trauma patients with essentially no risk of intracranial injury on imaging has been a priority among ED physicians.6,7,30 Such a tool would allow significant reductions in health care costs while also reducing patient exposure to radiation exposure. It is also important to ensure that any set of imaging decision rules is sensitive enough and has a high negative predictive value not to miss any cases of intracranial injury that would require neurosurgical intervention or lead to significant disability. It is important that any guidelines that are developed for CT ordering mTBI be easy to use for clinicians and easy to remember. It is also important that education of clinicians to the benefits of the rules be thorough and efficient, indicating the advantages of limiting overuse of CT in the ED, as well as the reliability and sensitivity of the implemented diagnostic algorithm for clinically significant intracranial injury. Typically, guidelines that are less complex and incorporate concrete definitions are easy to follow and have strong supporting evidence have higher rates of compliance. Four clinical decision-making criteria have been developed to guide CT imaging in the setting of mTBI, aimed at including all cases of clinically significant intracranial imaging while also reducing unnecessary CT imaging. These include the Canadian CT Head Rules (CCHR), the New Orleans Imaging Criteria (NOC), the National Emergency X-Radiography Utilization Study II (NEXUS II), and the American College of Emergency Physicians (ACEP)/Centers for Disease Control and Prevention (CDC) Clinical Policy Recommendations. The CCHR Criteria were developed from the initial evaluation of 3121 adult mTBI patients who had been treated in the ED of 10 major Canadian hospitals.11 Inclusion criteria were age greater than 16, blunt head trauma resulting in witnessed LOC, the presence of amnesia or witnessed disorientation, initial ED GCS score of 13 or greater, and injury within 24 hours at the time of evaluation in the ED. Of the patients evaluated, 8% had clinically significant intracranial injury as determined by CT imaging (i.e., injuries that would require hospitalization and neurologic follow-up), and 1% required neurosurgical intervention. An additional 4% were also found to have clinically unimportant intracranial injuries, specifically focal subarachnoid hemorrhages or cerebral contusions measuring less than 5 mm in neurologically intact patients. Based on this evaluation, a clinical decision rule was developed that included five high-risk factors for neurosurgical intervention: GCS lower than 15 at 2 hours after the initial injury Suspected open or depressed skull fracture Any sign of basal skull fracture “Raccoon” eyes Hemotympanum Cerebrospinal fluid otorrhea/rhinorrhea Battle’s sign Vomiting at least twice Age 65 years or older If any of these factors are present, there is a high likelihood of need for neurosurgical intervention. In addition, two medium-risk criteria were identified that portended clinically important intracranial injury without the need for neurosurgical intervention. These included dangerous mechanism of injury (e.g., pedestrian struck by a motor vehicle, a fall from greater than 3 feet or five stairs, being ejected from a motor vehicle) and amnesia after impact for longer than 30 minutes. The high-risk criteria had a sensitivity of 100%, identifying all 44 cases that required neurosurgical intervention, as well as a specificity of 68.7%. The CT imaging rate was 32%. When the high- and medium-risk criteria were considered, the sensitivity and specificity were 98.7% and 49.6%, respectively, with a CT imaging rate of 54%. Cranial CT imaging was considered mandatory in patients who fulfilled the high-risk criteria and was recommended in medium-risk patients. One important feature of the criteria is that they considered certain intracranial injuries as being not clinically important, specifically, a solitary contusion smaller than 5 mm, localized subarachnoid hemorrhage less than 1 mm thick, subdural hematoma measuring less than 4 mm, pneumocephalus, and closed depressed skull fracture not involving the inner table. Minor head injury is defined as witnessed LOC, definite amnesia, or witnessed disorientation in patients with a GCS score of 13 to 15. Stiell et al31 applied their CCHR prospectively at 12 different academic and community radiology practices in Canada and evaluated changes in ordering ED CT head imaging ordering before and after implementation of these rules. Six institutions were randomized into “intervention” sites, at which the CCHR were implemented, whereas six others served as “control” sites, in which there was no implementation or education of these rules. Between the two groups, in the evaluation of a total of 4531 patients, no cases were included in which clinically significant intracranial injuries were not imaged; however, the CT ordering rate increased at both the control and intervention sites between before and after implementation of the rules. At the intervention sites, the rate of use increased from 62.8 to 76.2% [a difference of 13.3% with 95% confidence interval (CI) of 9.7 to 17%), whereas the control sites showed an increase of 67.5 to 74.1% in use (a difference of 6.7%, with 95% CI of 2.6 to 10.8%).31 This study again confirmed that the CCHRs have high sensitivity for clinically important intracranial injury, but implementation of these rules did not reduce, but rather increased, CT imaging use relative to control and baseline use. According to the authors, this result may have been secondary to inadequate education before rule implementation, an inability of the ED physicians to recall the rules, abnormally low use rates at the participating institutions before intervention, and a lack of compliance on the part of the ED physicians to implement the rules for a myriad of reasons.31 Other reasons for this discrepancy could be the differences in health care systems between Canada and the United States and the lesser availability of CT scanners in Canada (only 30% of urgent care settings had CT scanners at the time) affecting utilization.32 According to other trials, implementation of the CCHR can potentially decrease CT use in the ED by up to 37%.5,33 U.S. health care savings estimates with appropriate implementation of this rule range from 120 to 400 million dollars annually.33,34 Haydel et al.2
2.1 Introduction
2.2 Who Requires CT Imaging?
2.2.1 Canadian CT Head Rules Criteria
2.2.2 New Orleans Imaging Criteria
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree