Jaime All • John F. Angle
Embolotherapy for extremity trauma is a well-established and essential tool for the management of hemorrhage and vascular injury with potential for hemorrhage. Familiarity with clinical presentation, pretreatment imaging, and angiographic findings are all essential components to interventionalists providing these services. Embolization technique and the similarities and important differences between pelvic and extremity injury will be discussed.
PELVIC TRAUMA
Within the context of a multispecialty approach to the care of the trauma patient, the transcatheter arterial embolization (TAE) is an essential tool in the treatment of traumatic pelvic arterial injury.1 Many trauma centers have developed algorithms based on the nature of the injury, computed tomography (CT) findings, and other injuries.2–5 Some trauma scenarios are best treated with TAE first, others are supplemented with TAE, and many will not benefit from early pelvic angiography.2–7 Blunt or penetrating pelvic trauma may lead to vascular injury, with blunt injuries being much more common in most geographic areas. Blunt trauma resulting in pelvic fracture is associated with vascular injury in up to 40% of patients, and hemorrhage is still the highest cause of mortality (50% to 74% of patients).8–10 With blunt pelvic injury, the source of hemorrhage may be venous, arterial, or from cancellous bone.7,11 TAE is only indicated for the treatment of arterial hemorrhage and is indicated in approximately 3% to 10% of cases.2,8,12,13 In the correct clinical setting, angiography with TAE is effective in controlling pelvic hemorrhage in 85% to 97% of cases.6 Patients embolized within 3 hours of arrival have a statistically improved survival rate as compared to those receiving intervention outside of the initial 3-hour window.14
Indications for TAE in the setting of pelvic trauma include pelvic fractures and hemodynamic instability after exclusion of other sources of hemorrhage outside of the pelvis, active extravasation of contrast on computed tomography angiography (CTA) in the presence of pelvic fracture regardless of hemodynamic status, prior TAE for pelvic trauma with ongoing hemodynamic instability after other sources of hemorrhage have been excluded, and patients older than 60 years of age with a high-risk pelvic fracture pattern.6,12,15,16
Motor vehicle collisions and pedestrians hit by motor vehicles account for most blunt arterial injury.12 Domestic falls are a less common cause, but the need for pelvic vascular injury evaluation in this population is well documented.7,12,16 This is particularly true for the elderly because age is an independent predictor of active bleeding and indication for TAE, regardless of hemodynamic stability.12,16 Additionally, osteoporosis in elderly women may result in increased rate of pelvic fracture, even when the mechanism of injury is less dramatic.17 The prevalence of chronic anticoagulation for cerebrovascular and cardiovascular disease in this patient population may also necessitate early intervention.
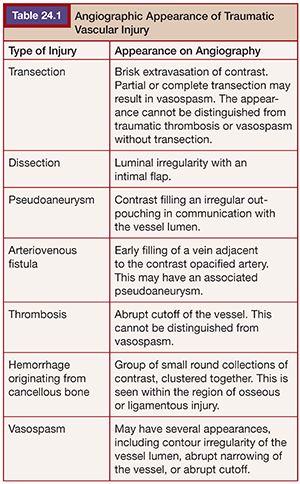
Types of vascular injury seen with blunt pelvic trauma include complete or partial transection, arteriovenous fistula, pseudoaneurysm, dissection, and traumatic thrombosis. The different angiographic appearances of traumatic vascular injury are listed in Table 24.1.1,18–20 Vascular injury typically occurs in relationship to adjacent osseous fractures or shearing forces against the ligamentous structures of the pelvis.19 It is important to note that disruption of the osseous pelvis significantly enlarges what is normally a confined space. This allows for a larger volume of hemorrhage to accumulate rapidly, and thus early intervention is necessary to prevent exsanguination.7 It is worth repeating that pelvic hemorrhage is usually from venous or fractured cancellous bone, and this type of low-pressure hemorrhage is best controlled with external fixation or compression with pelvic binding. The goal of these methods is to reestablish the normal tamponading effect of the retroperitoneum, although some recent studies call into question the overall efficacy with this approach.6,19,21 Peritoneal pelvic packing has been introduced as an additional method of treating pelvic hemorrhage. Some centers have questioned; whether pelvic packing should replace TAE, most use TAE as an adjunctive treatment, and others suggest TAE first with fixation secondarily.2–7,21 From a practical standpoint, we push for angiography first if active extravasation is seen on CT and suggest deferring the fixation, which can make angiography very difficult to perform. Regardless of the algorithm or combinations of treatment employed, early control of pelvic arterial hemorrhage has a strong association with improved outcome, and TAE continues to occupy a central role in the treatment of traumatic pelvic hemorrhage.1–6,14
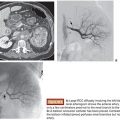
As many as 50% of patients with pelvic fracture have additional injuries, so pelvic fracture should be treated as an indicator of polytrauma (Fig. 24.1).21 An algorithm approach to the care of the trauma patient, although institution-dependent, must weigh the severity of multiple injuries and therefore mandates a multidisciplinary approach. Most would prioritize an intra-abdominal source of hemorrhage over intrapelvic bleeding, although a few have argued to the contrary.4,6,22 However, it is accepted that exploratory laparotomy in the setting of unaddressed pelvic hemorrhage can lead to rapid expansion of the pelvic hematoma.9 Additionally, some solid organ injury within the abdomen may be treated at the time of pelvic embolization, such as a splenic or renal laceration. Regardless of when TAE is performed, consideration must be given to the possibility of additional organ injury, and this should guide the goal of the procedure in terms of the degree of vessel selectivity. Failure of hemodynamic status to correct following embolization should prompt search for additional sources of bleeding.
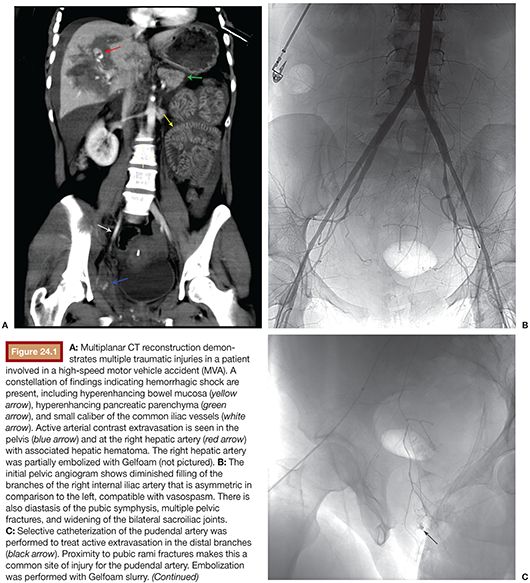
Historically, fracture patterns were felt to be important diagnostic indicators in pelvic trauma.9,19 More recent studies have shown that arterial hemorrhage can occur with any of the fracture patterns, as well as in the less severe subtypes, and may occur in the absence of fracture.22 Those most worrisome (and those which are considered high-grade at our institution) are vertical shear and types II and III lateral compressions and anterior posterior compressions.9,19,23 It is still important for the interventionalist to recognize these patterns because they may suggest which vessel is the culprit, even in the absence of active extravasation at angiography. At the most basic level, anterior fracture patterns are associated with injury to the anterior division of the internal iliac artery, and posterior fractures are associated with injuries to the posterior division.1,23 The importance of fracture pattern recognition has been largely supplanted by CT, which has superior diagnostic sensitivity, and adds a wealth of additional data over conventional radiography.24,25 Advances in technology have allowed for rapid image acquisition and processing, thereby allowing for the imaging of patients whose status may be tenuous and, in some centers, even those who are unstable.
Examples of penetrating pelvic injuries include gunshot wounds, stab wounds, or iatrogenic injury (such as with percutaneous drainage tube placement and placement of orthopedic hardware).26 Penetrating injuries are more likely to result in transection or pseudoaneurysm formation, with or without arteriovenous fistula.27 Traumatic pseudoaneurysms are at high risk for rupture and should all be treated, preferably with endovascular methods when possible.18
EXTREMITY INJURY
Traumatic vascular injury to the extremity may also cause life-threatening hemorrhage, but, more commonly than in the pelvis, it may result in vessel occlusion with signs of acute limb ischemia. Iatrogenic injury accounts for 74% of embolization procedures performed in the extremity.28 Many of these are associated with orthopedic procedures, which are likely to increase as our aging population requires an increasing number of major joint procedures. Arterial injury associated with orthopedic procedures typically involves a branch vessel in the pelvis or lower extremities, rather than a major artery, and is thus ideally suited to treatment via endovascular methods.28
Traumatic arterial injury of the extremity is divided into penetrating or blunt trauma. The distinction between the two has important clinical implications. Penetrating injury (more common) results in pseudoaneurysm, arteriovenous fistula, and partial or complete transection.29 Outside of iatrogenic causes, glass is the most common source of penetrating injury followed by gunshot wounds and stab wounds.30 Blunt injury is often seen with associated orthopedic trauma such as fracture and dislocation. The vascular injury seen with blunt trauma is typically dissection, pseudoaneurysm, or occlusion secondary to intramural hematoma or traumatic thrombosis.31 Diagnosis of arterial injury in the setting of a penetrating mechanism is usually more straightforward as there is obvious external evidence of injury. Hard signs of injury are more likely to be present, such as active external hemorrhage, enlarging hematoma, or obvious indicators of limb ischemia.27 Signs of limb ischemia include pallor, abnormal peripheral pulses, unequivocal neurologic defects, and a thrill or bruit.32
Blunt peripheral arterial injury is more likely to be occult with delayed diagnosis and concomitant traumatic injuries to the head and body, which may take precedence over the extremity.31 Diagnosis of blunt arterial injury requires a higher index of suspicion and recognition of soft signs of vascular injury. These include (1) discrepancies in the ankle–brachial index performed with Doppler ultrasound and (2) adjacent injury, including fracture, nerve injury, or stable hematoma.18 Duplex ultrasonography may be beneficial for characterization of some specific vascular injuries, but CTA provides clinically important additional information about extremity osseous and soft tissue structures.27,33 Additionally, newer CT scanners can acquire arterial phase imaging of the extremities during the concomitant CTA evaluation of the chest, abdomen, and pelvis. Conventional angiography for diagnostic evaluation of acute arterial injury has largely been replaced by CTA, given the excellent sensitivity of CTA.33
Vascular injury is more common in the lower extremity than the upper extremity, and the femoral and popliteal arteries are the most common sites of vascular traumatic injury.34 Knee dislocation is a common cause of occult lower extremity arterial injury.32 In the upper extremity, the brachial artery is the most commonly injured vessel followed by the radial and ulnar arteries.30 The arteries of the forearm typically do not require intervention for occlusive traumatic injury secondary to collateral flow at the palmar arch.30 Blunt brachial artery injury is most commonly seen in children in the setting of elbow dislocation or displaced supracondylar fractures.35 Injury to the axillary artery is less common but can be seen with severe anterior dislocation of the glenohumeral joint.31 Additional damage to the branch vessels of the axillary artery can also be seen with this mechanism of injury.
With respect to extremity treatment, embolotherapy is predominantly indicated for hemorrhage originating from branch vessels that do not provide distal perfusion.18,29 Pseudoaneurysms and arteriovenous fistulas, even those without active extravasation, are often appropriate indications for embolization as well.18,29 Acute injury of the larger vessels is more often treated surgically, although endovascular treatment with stent graft placement is increasing.36 Branches of the profunda femoral artery are an ideal site for endovascular treatment of acute traumatic hemorrhage because these do not provide direct perfusion to the distal extremity and are traditionally difficult to access via a surgical approach.19 The branches surrounding the shoulder and the profunda brachial artery are similarly potential candidates for TAE in the upper extremity.28,37
TECHNIQUE
Preprocedural Planning
Familiarity with the trauma history and all available medical information including prior imaging is essential to assisting the trauma team in assessing the appropriateness and timing of angiography. The local algorithm for the treatment of the trauma patient should also be clearly understood. The interventionalist should be familiar with Advanced Trauma Life Support (ATLS) protocol and have an appropriate level of critical care support in the angiography suite.
Embolic materials used in trauma are almost always coils, microcoils, or Gelfoam (Pfizer Inc., New York, New York). Alternative large vessel occlusion devices such as the Amplatzer 4 Vascular Plug (St. Jude Medical, Inc., St. Paul, Minnesota) have seen increasing usage. Some authors have described the use of liquid embolic agents such as Onyx (Covidien, Irvine, California) and N-butyl cyanoacrylate (Trufill; Codman & Shurtleff, Inc., Raynham, Massachusetts), with the potential advantages of achieving vessel occlusion in the setting of coagulopathy and providing distal control when distal catheterization is not possible.38,39 Cost, a steep learning curve, and a potentially narrow safety profile are noteworthy considerations in the use of liquid embolic agents. Catheter-directed thrombin has also been described for the treatment of traumatic pseudoaneurysms of branches of the internal iliac artery.40 We feel that the efficacy and safety profile of autologous clot, Onyx, or glue are better choices when distal control cannot be achieved. Small particles, including Gelfoam particles, have little or no role in the treatment of acute trauma, and the use of alcohol in the trauma patient is contraindicated.18,19 Additional embolic agents that are mostly of historical interest include clipped pieces of silk suture and segments of the outer winding of a 0.035-in Bentson guidewire (Cook Medical, Inc., Bloomington, Indiana).
Periprocedural considerations should include anticipating the impact of CTA contrast within the urinary bladder. If there is suspicion for urethral injury, pelvic angiography should not be delayed and consideration should be given to delaying cystography until after the embolization procedure to avoid extravasation of contrast.19
Examination of the extremity color, temperature, and pedal pulses (radial and ulnar pulses if upper extremity access is planned) should be assessed by the interventionalist performing the procedure. Any postprocedure change should prompt query into a potential complication, such as reflux of embolic agents into the extremity vessels or access site vascular injury.
SITE OF ACCESS AND DIAGNOSTIC IMAGING
Pelvic binders vary among institutions. Some are made of fabric, and a hole can be cut through them for femoral access. Others are leather binders, which may have to be undone before obtaining access. Most pelvic embolizations can be performed via a single common femoral artery access site. If there is known unilateral pelvic or lower extremity injury, contralateral femoral access is preferred. If injuries or external devices preclude the preferred femoral access, the procedure can be performed via a brachial or even popliteal or radial artery access. More recently, dual access has been advocated to allow placement of a temporary occlusion balloon within the infrarenal abdominal aorta while pelvic or lower extremity hemorrhage is treated.7,11 We advocate the use of ultrasound guidance for all arterial punctures. When used routinely, it adds little time to the procedure and avoids the complications encountered with suboptimal access (e.g., retroperitoneal hematoma or arteriovenous fistula). This is especially important when dealing with hemorrhagic shock, as these patients already have little cardiovascular reserve and may not tolerate iatrogenic injury superimposed on traumatic injury. Ultrasound guidance also increases the likelihood that a closure device can be successfully used at the end of the procedure.
Often in the acute setting, renal function has not yet been assessed, and the risk of contrast nephropathy becomes a secondary concern. In a patient with known renal insufficiency, nonselective and selective pelvic and lower extremity angiography may be performed with carbon dioxide (CO2).
The procedure should start with nonselective angiography to quickly localize sites of hemorrhage and serves as a road map for vessel selection. Additionally, the initial imaging is useful in differentiating traumatic injury from guidewire-induced vasospasm. Fractures should prompt selective angiography at potential sites of vascular injury. For instance, the superior gluteal artery is the most commonly injured vessel with a posterior fracture pattern, and this is typically injured as it passes under the sciatic notch. It is commonly injured with open book fractures.19
Appropriate angiographic imaging of the internal iliac artery requires orthogonal selective views. The anterior division is best imaged with a contralateral oblique view of approximately 45 degrees. This can be thought of as looking at the iliac wing en face. An ipsilateral anterior oblique view is important to identify the superior gluteal artery and the internal pudendal artery. It should be stressed that, although initial nonselective pelvic angiogram is an important part of pelvic or extremity evaluation, selective angiography is crucial to achieving appropriate angiographic sensitivity.
Hemorrhage from pelvic trauma is most often associated with the internal iliac artery, but some have advocated that a selective angiogram of the external iliac artery should also be obtained.41 Although the external iliac artery is not usually a source of pelvic hemorrhage, the external pudendal, deep iliac circumflex, inferior epigastric, and the circumflex femoral arteries may be injured.1,3,42 The more inferior lumbar arteries as well as the inferior mesenteric or even the gonadal arteries are other rare sources of pelvic bleeding.
Following completion of contralateral pelvic vessel evaluation, selection of the ipsilateral common iliac artery should be obtained with a reverse curve catheter or a tightly curved catheter such as the Rim catheter (AngioDynamics, Latham, New York). Imaging of the bilateral iliac vessels should always be obtained. This avoids continued hemorrhage resulting from reperfusion of the injured vessel via anastomoses with the contralateral pelvic arteries. It also rules out additional injury, which may have been occult on initial CT imaging.
Pelvic Embolotherapy
The decision to embolize should be based on angiographic findings of vessel injury but not limited to active extravasation. This aggressive approach may even include embolization of potential sources of hemorrhage identified on CT. Less selective or nonselective internal iliac embolization is recommended by some if the patient is hemodynamically unstable or if multiple sites of active extravasation are identified. Nonselective embolization of the internal iliac arteries is performed with the injection of Gelfoam, often followed by coils with the tip positioned in the internal iliac artery. Nonselective embolization is probably not appropriate for most pelvic trauma and is not appropriate for the profunda artery given the risk of ischemia. At the very least, CT will usually direct the operator to nonselectively embolize either the anterior or posterior division of the internal iliac artery.
Gelfoam, widely considered the workhorse of trauma embolotherapy, is a temporary agent that allows for potential recanalization within a few weeks. This may allow for reclaimed tissue perfusion once the acute injury has had time to heal. It is also inexpensive and readily available. Gelfoam is typically delivered as slurry. This is mixed in a three-way stopcock with a half-strength mixture of contrast and normal saline. More agitation will create a Gelfoam mixture that is less coarse. If the target of embolization with Gelfoam is distal, the Gelfoam mixture should be kept coarse. This achieves hemostasis through occlusion proximal to the end capillary bed, thereby mitigating against the complication of tissue necrosis.6,43 Gelfoam can also be delivered in the form of 2- to 3-mm pledgets termed torpedoes. This is achieved by front-loading a 2- to 3-mm piece of Gelfoam into the tip of a 1-mL syringe of half-strength contrast. The torpedoes may be soaked in contrast before injection. This can be used to achieve occlusion of larger caliber vessels and may reduce the risk of ischemia. The delivery of Gelfoam should be followed with a contrast injection after every few 0.1- to 0.3-mL injections of slurry or every few torpedoes, with the goal of sluggish flow and not complete stasis of contrast.19,29 This technique helps limit reflux of embolic agents into other branches.
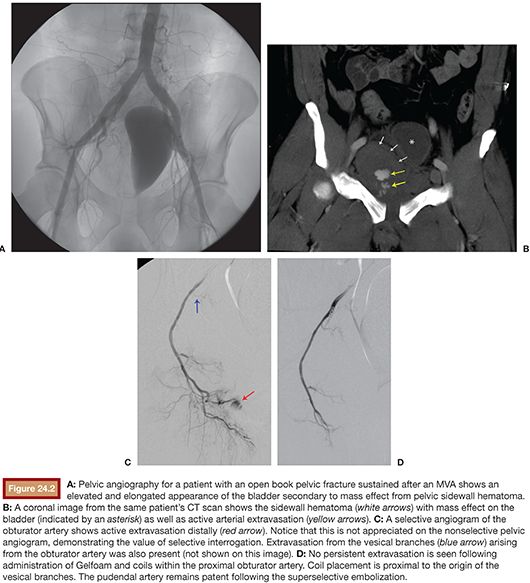
The administration of Gelfoam into internal iliac artery branches is typically followed by selective branch occlusion with coils (Fig. 24.2). The theory is that the coils prevent very early recanalization of the vessel but are proximal enough to not cause ischemia. The problem with coils is that they may block access to bleeding vessels if repeat embolization is needed. If a 4-Fr or 5-Fr catheter has been advanced to the target vessel, then 0.035-in coils such as a Nester or Tornado (Cook Medical, Inc., Bloomington, Indiana) can be used. These 0.035-in coils have Dacron fibers to promote thrombogenesis. The 0.035-in coils should be pushed and not injected. Superselective catheterization of the target vessel is typically accomplished with a 0.021-in lumen 3-Fr microcatheter (e.g., Cantata; Cook Medical, Inc., Bloomington, Indiana). A high-flow microcatheter, which typically has a 0.025-in lumen, such as the Progreat Omega (Terumo Medical Corporation, Somerset, New Jersey), provides the ability to perform rapid power injection. The slightly larger inner lumen introduces concerns with compatibility of 0.018-in microcoils (e.g., Nester or Tornado) with high-flow microcatheters because coils can fold over and become wedged within larger lumen microcatheters. This will potentially make arterial branch access difficult for the operator to obtain.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree