Fig. 17.1
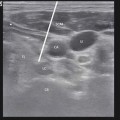
Spine model where a lumbar facet joint is located within a white circle, and the course of a medial branch bifurcating after crossing the transverse process is shown to contribute to a dual innervation pattern
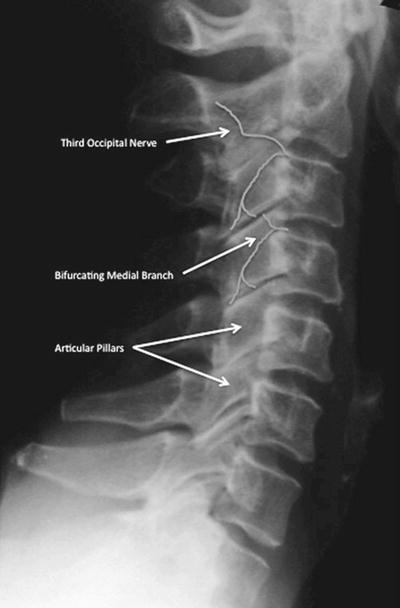
Fig. 17.2
Lateral radiograph of cervical spine identifying the articular pillars, demonstrating the bifurcation of the medial branch at C4 for a dual innervation pattern and the course of the third occipital nerve
The thoracic facet joint innervation has a pattern similar to that of the lumbar region, except for findings from a study of four cadavers that demonstrated consistency of the medial branch course at the superolateral aspect of the transverse processes. The medial branches at these levels travel lateral from the foramen, cross the superior lateral border of the transverse process, and course medial to innervate the corresponding facet joint and level below. However at the T5–T8 levels, the inflection point of the nerve occurs at a point just superior to the superolateral corner of the transverse processes [15].
Pathophysiology
Intervertebral disc space narrowing occurs as the disc degenerates and loses hydration. The change in segment height can cause subluxation of the facet joints, resulting in abnormal stresses on the joint and nerve root impingement. Other sequelae, such as capsular irritation and local inflammation, may result in reflex spasm of the erector spinae muscles. As degeneration proceeds, abnormal motion leads to osteophyte production, further exacerbating the symptoms [16]. That the facet joint is a source of nociception has yet to be universally accepted. Opponents submit that local anesthetic blockade of the facet joint with subsequent pain relief lacks validity. This position is supported by observations of contrast injection spilling over into the epidural space or intervertebral foramen [7]. Pain elicited with hypertonic saline or relief with local anesthetic administration may be due to action on neural structures or on other pain-sensitive tissues. Proponents for the facet joint as a site of nociception point to the presence of substance P in facet capsule neurons [17]. In addition, most of the mechanosensitive somatosensory units in the facet joint are group-III high-threshold, slow-conduction units, which are thought to mediate nociception [18–20]. Chronic inflammation may lead to fluid accumulation and distension, stimulating the richly innervated synovial villi inside the capsule, resulting in pain.
Facet Block: Diagnostic or Therapeutic Tool?
Lumbar facet arthropathy is characterized by low back pain, unilateral or bilateral, with or without radiation. The pain is described usually as a deep, dull ache; is difficult to localize; and frequently is referred into the buttock, groin, hip, or posterior thigh to the knee. Fukui et al. described referral patterns for thoracic facet joints [21].
Some patients describe a sudden onset of pain, usually associated with twisting or bending. There is no exacerbation of the pain with Valsalva maneuver. In contrast to discogenic pain, sitting does not severely aggravate pain secondary to facet arthropathy. The cervical facet joints also cause pain described as deep and aching. Referral patterns vary, depending on which level is of concern. The C1–C2 facet joint may refer pain to the occipital and postauricular region [22]. The C2–C3 facet joint may cause pain referred to the occiput, ear, vertex, forehead, or eye [23, 24]. The C3–C4 facet joint refers pain over the posterolateral cervical region, following the course of the levator scapulae. The lower cervical facet joints refer pain to the base of the neck and down to the scapulae (Fig. 17.3) [23]. Physical examination often reveals tenderness over the facet joints and involves associated muscle spasm. The pain is exacerbated by extension or lateral bending as opposed to flexion as well as prolonged sitting. A few patients may exhibit mechanical hyperalgesia over the associated innervated skin. Whereas range of motion in all directions may be reduced, extension and rotation are most uncomfortable. Straight leg raise is usually negative. To make the diagnosis of a painful facet joint requires the typical history and physical findings already described in combination with diagnostic blocks. The use of facet block for diagnosis is hampered by certain pitfalls. There is a lack of a corresponding cutaneous innervation to the facet joint and thus an inability to determine when complete blockade has occurred. Injection into the joint often results in joint capsule rupture and spillage of local anesthetic into the epidural space or intervertebral foramen, which can interrupt nociceptive impulses from alternative sites [20, 25–27]. The medial branch nerve innervates muscles, ligaments, and periosteum in addition to the facet joints, again limiting specificity of the test. Facet blocks should be avoided in patients with systemic infection, infection at the site, or coagulopathies or in patients who refuse the procedure. Needle placement for facet injection as well as local anesthetic delivery can result in pain provocation. A provocative response that is concordant with the patient’s ongoing complaints lends further support to the notion that the facet joint is the pain generator. Facet injections are commonly used for both therapeutic and diagnostic interventions. Intra-articular steroid injection often produces significant pain relief that outlasts the action of a local anesthetic [26, 28–30]. Although therapeutic benefit from steroid has been demonstrated, duration of outcomes is limited, similar to intra-articular steroids delivered to other joints [31–33]. Intra-articular block also does not correlate well with the success of radiofrequency denervation (only 64 %); therefore, medial branch block is the preferred procedure as a trial prior to facet denervation [34].
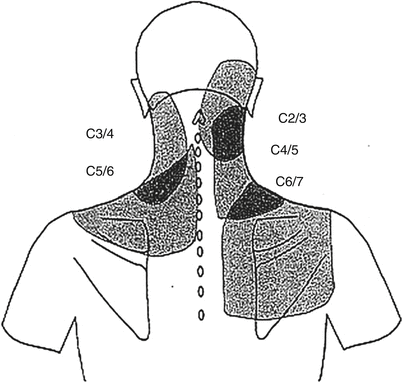

Fig. 17.3
The C3–C4 facet joint refers pain over the posterolateral cervical region, following the course of the levator scapulae. The lower cervical facet joints refer pain to the base of the neck and down to the scapulae
Technique
Lumbar and Thoracic Facet Blocks
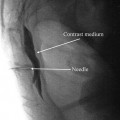
For facet joint injection, the patient is positioned prone, with an abdominal cushion to reduce lumbar lordosis. Sterile preparation and draping of the back are performed. Intra-articular injection requires oblique fluoroscopic views. Best results are achieved at a 30–45° plane to “open” the joint. Either the table or the C-arm can be rotated for optimal viewing. The entry point through the skin then is identified and marked with the aid of a radiopaque instrument. The skin is infiltrated with 1 % lidocaine using a 25-gauge needle. A 22-gauge, 3.5-in. spinal needle then is introduced via the skin wheal and advanced into the joint using a trajectory parallel to the fluoroscopy beam. Local anesthetic alone or with steroid (0.25 % bupivacaine and 20 mg Depo-Medrol (methylprednisolone acetate)) is delivered in a volume of 1.0–1.5 mL. Volumes in excess of 2 mL will rupture the capsule and spill over into the epidural space (Fig. 17.4). For medial branch block, the patient is positioned prone, and the transverse process for each branch to be blocked is identified using fluoroscopy. Approximately 5 cm from the midline, a skin wheal is raised, and a 22-gauge, 3.5-in. spinal needle is advanced to the medial end of the transverse process, contacting the dorsal surface of the process near the superior edge. The L-5 medial branch is blocked at the groove between the ala of the sacrum and the superior articular process of the sacrum (Fig. 17.1). A total volume of 1.0 mL of 0.5 % bupivacaine is delivered at each site, and the patient is questioned for concordance compared with the original pattern of referred pain (Fig. 17.5). The technique is slightly altered for the thoracic levels, where the superolateral aspect of the transverse processes is the ideal site for placement.
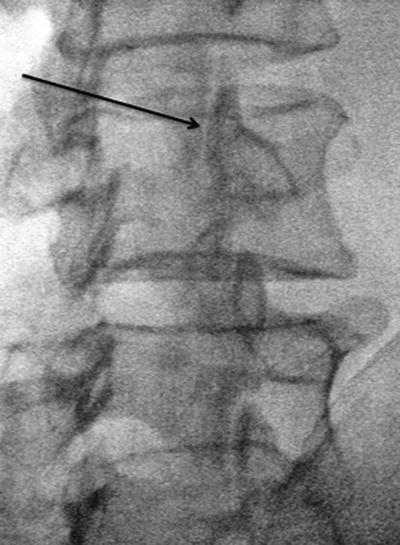
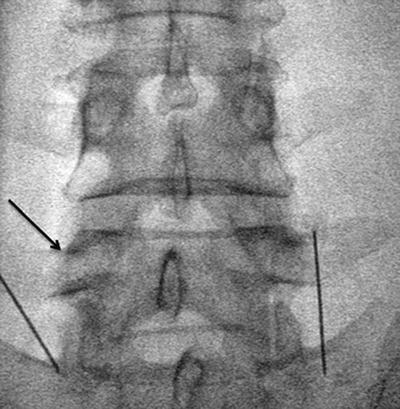

Fig. 17.4
“Scotty dog” view of facet joint at a 30° angle for intra-articular injection

Fig. 17.5
AP view of lumbar facet medial branch block with needle at groove of sacral ala and arrow pointing to desired placement at superomedial aspect of the L5 transverse process
Cervical Facet Block
The patient is ideally positioned prone to reduce potential injury to the vertebral arteries, but a lateral position has also been described. Sterile technique and needles are used as previously outlined. Needles are introduced 1–2 cm lateral to the waist of the articular pillar, guided by a posteroanterior view on fluoroscopy. The needle then is advanced to the centroid of the articular pillar as seen on a lateral view (Figs. 17.6 and 17.7). Again, 1.0 mL of local anesthetic is delivered. Intra-articular injection at the cervical level is not favored for several reasons. Cervical joint spaces are small and narrow. Further, the epidural space is immediately medial to the joint, and the vertebral artery is just lateral to the joint. Therefore, direct injection into cerebral circulation or blockade of cervical nerve roots is of great concern.
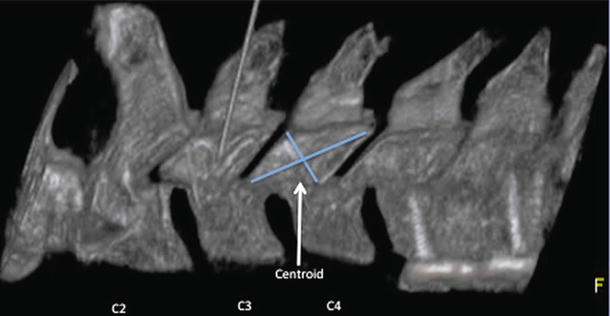
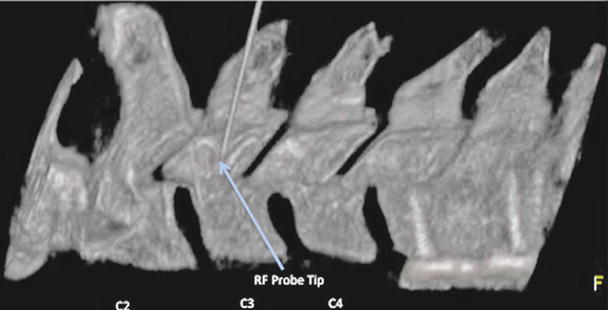

Fig. 17.6
With a lateral view of the cervical articular pillar, the intersection of lines connecting the opposite corners locates the centroid of the articular pillar where the medial branch typically will be found

Fig. 17.7
Lateral view of 3-D reconstructed image demonstrating needle placement at the centroid of the C3 articular pillar
Facet Joint Denervation
Denervation of the medial branch can be accomplished by using either radiofrequency ablation or cryoneurolysis. This chapter will focus on the radiofrequency method in terms of its mechanism of action, followed by the results of long-term outcome studies.
Conventional Radiofrequency Ablation
The radiofrequency lesion generator has the following critical functions: (1) continuous online impedance measurement; (2) nerve stimulation; (3) monitoring of voltage, current, and wattage during radiofrequency lesioning; and (4) temperature monitoring. Electric impedance is measured to confirm the continuity of the electric circuit and to detect short circuits. Impedance is usually 300–500 Ω in extradural tissue. The nerve stimulator is used to detect proximity to sensory or motor fibers of the segmental root. Stimulation at 50 Hz is used to detect sensory fibers; 2 Hz is used to detect motor fiber stimulation. Ford et al. demonstrated that if the electrode is resting on the nerve, 0.25 V will be required to produce discharge, whereas 2 V will be required to produce discharge at a distance of 1 cm [35]. Therefore, monitoring voltage is important in determining proximity. Temperature monitoring occurs at the tip of the electrode only, with a thermocouple technique, producing a thermodionic voltage that is proportional to temperature. Bogduk et al. performed lesions in egg whites and meat and found that radiofrequency lesions do not extend distal to the electrode tip. Instead, lesions extended radially around the electrode tip in the shape of an oblate spheroid with a maximal effective radius of 2 mm using a 21-gauge electrode with a 3-mm exposed tip [38]. Table 17.1 demonstrates a survey of varying tip sizes and temperatures with the corresponding lesion size. The first signs of coagulation occur at 62 °C, but it is important to note that neural destruction begins at 45 °C. The maximal lesion size is attained once the “working” temperature is maintained for 20–40 s. Maintaining the temperature for longer periods did not result in any discernible increase in lesion size [39]. Although initial reports indicated selectivity for small fibers, Uematsu conclusively showed that radiofrequency at higher temperatures indiscriminately damages both small and large fibers [40]. Placement of the probes requires positioning of the active tip along the course of the medial branch as previously described (see Figs. 17.8 and 17.9).
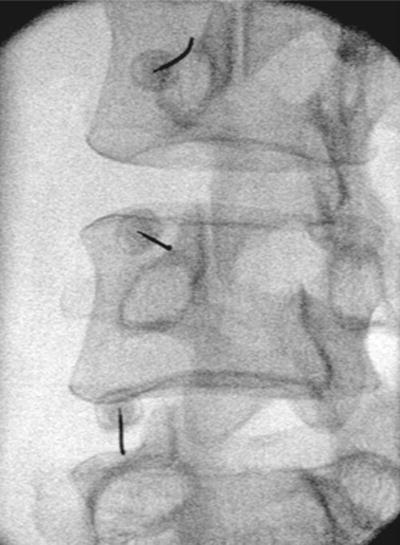
Table 17.1
Relationship of lesion size with tip sizes and temperatures
Authors | Electrode diameter (mm) | Exposed electrode tip length (mm) | Tip temperature (C) | Transverse lesion size (mm) | Test medium |
|---|---|---|---|---|---|
Bogduk et al. [38] | 186 | 5 | 80 | 2.2 ± 0.4 | Egg |
226 | 4 | 80 | 1.1 ± 0.2 | Egg | |
226 | 4 | 90 | 1.6 ± 0.2 | Egg | |
Cosman et al. [36] | 216 | 3 | 65 | 2–4 | Egg |
Guy, et al. [37] | 216 | 2 | 60 | 3.7 | Rabbit cortex |
216 | 2 | 70 | 5.5 | Rabbit cortex | |
216 | 2 | 80 | 7.2 | Rabbit cortex | |
Vinas et al. [39] | 206 | 4 | 80 | 4.9 | Rabbit cortex |

Fig. 17.8
Oblique view demonstrating placement of RF cannulae after contact with the “eye” of Scotty dog and slipped off the superior margin of the transverse processes

Fig. 17.9
Lateral view of placement of RF cannulae along the lumbar superior articular processes
Retrospective studies of lumbar facet RF denervation have demonstrated similar rates of success. Goupille et al. showed a 38.4 % success rate at 2-year follow-up, and North showed a 45 % success rate with a mean follow-up of 3.2 years [41–43]. North et al. went further, concluding that there was no difference in success for bilateral denervation for bilateral pain compared with unilateral denervation for unilateral pain [43]. Goupille et al. reported that patients who did not have prior spine surgery had better success with denervation, whereas North’s group did not show any statistical difference between these groups (Table 17.2). Van Kleef et al. performed a lumbar facet RF denervation double-blinded RCT in 31 patients with 80 C lesions at L3–L4, L4–L5, and L5–S1 with a sham control. At 8 weeks, mean VAS score was 4.8 for controls and 2.8 for the treated group. This was statistically significant for both differences in VAS but for Oswestry scores as well. In the treated group, 10/15 patients were successfully treated (at least 2-point reduction on VAS and greater than 50 % pain relief) at 8 weeks, and of these patients, seven were still a success at 12 months [44]. Nath et al. performed a sham-controlled RCT of lumbar facet RF denervation in 40 patients after at least 80 % pain relief was documented from controlled medial branch blocks. The RF group had multiple lesions performed at each level. At 6 months, the RF group had statistically significant improvement in VAS scores and with the patients’ global assessment in comparison to the sham group. There was also significant improvement in secondary measures such as spine range of motion, quality of life measures, and physical exam findings posttreatment [45]. A 10-year prospective clinical audit of lumbar facet RF denervation in 209 patients was able to have 2-year follow-up data on 174 of the patients. Of these individuals, 119 (68.4 %) had good (>50 %) to excellent (>80 %) relief at 6 months. At 12 months, 81 patients still had good to excellent relief, and this was maintained in 36 patients at 24 months (Table 17.2).




Table 17.2
Series showing lumbar facet RF denervation
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree