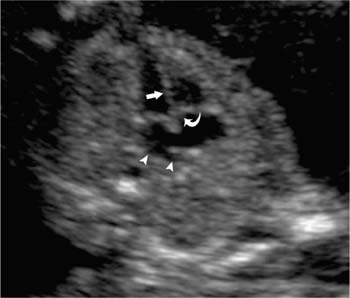
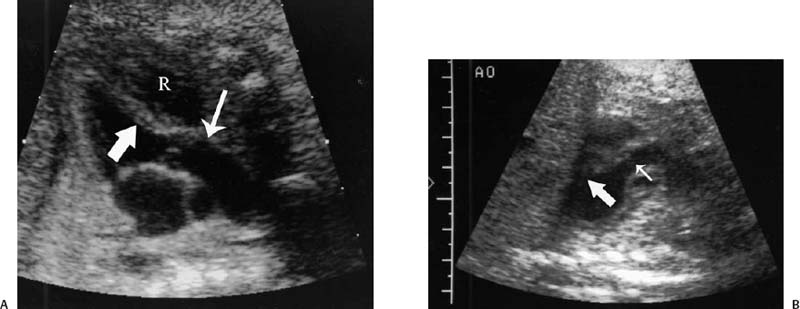
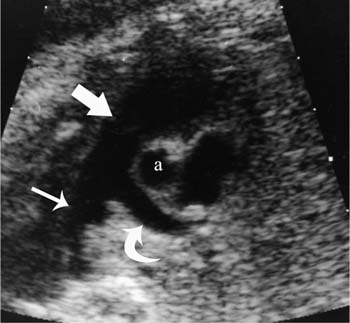
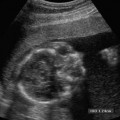
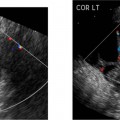
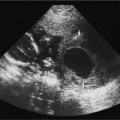
16 Family History of Congenital Heart Disease Congenital heart disease (CHD) is estimated to occur in 0.4 to 0.8% of live births.1,2 Because the majority of fetuses with CHD do not have a known risk factor,3–5 it is important to evaluate the heart of each fetus who has a sonogram. However, there are fetal and maternal factors that increase the risk of CHD. Fetal risk factors include a thick nuchal translucency, hydrops, extracardiac anomalies, complete heart block, and single umbilical artery. Maternal risk factors include metabolic disorders, teratogens, and a family history of CHD.6 Diabetes mellitus and phenylketonuria are metabolic disorders that predispose to CHD. Teratogens that are associated with CHD include lithium, anticonvulsants (such as phenytoin, trimethadione, and valproic acid), ethanol, and retinoic acid. Although ~10 to 15% of CHD is considered to be due to chromosomal abnormalities (such as trisomy 21, 18, and 13, or Turner’s syndrome), genetic syndromes (such as Noonan’s or Williams syndrome), or teratogens,6 the cause of the majority of cases of CHD is not well understood7,2 and is believed to be multifactorial.9 A family history of CHD will generate concerns for recurrence and typically prompts evaluation of the fetal heart in subsequent pregnancies. It is useful to know as much information as possible about the previous affected family member and the full family history, however, because this will influence the recurrence risk. CHD that is isolated carries a different significance than if the affected family member had a chromosomal abnormality, a syndrome associated with CHD, or knowledge that the CHD previously was related to teratogen exposure. In the absence of any of these other associations, there are some general risk estimates available. Most of the available data relates to affected first-degree relatives. The recurrence risk in a particular family is not always clear, but in general there is an increased risk for recurrence of CHD when either parent or a previous child has CHD. The recurrence risk when one previous child has CHD is estimated at 2 to 5%,1,7,8 suggesting a 3- to 10-fold increase over the baseline population risk of CHD. If two previous children have had CHD, the risk increases slightly to 3 to 10%.7,2 If a parent has CHD, the recurrence risk for the fetus has been variably reported, ranging from 1.5 to 17.8%.2,10–12 Many of the prior studies did not have a control group and could not estimate relative risk. A more recent study with a control group found a 3.1% prevalence of CHD when a parent had CHD, with an adjusted odds ratio of 1.73 (95% CI 0.89 to 2.44).10 The risk of recurrence also appears to be higher when the mother, rather than the father, is the parent affected with CHD.10,11,13 It is important that a full family history be taken, however. If there have been multiple affected individuals in a family, the recurrence risk may be higher than what is predicted by population-based studies.1 The recurrence risk for second- and third-degree relatives of a single-index case with isolated congenital heart disease is probably not much different than the general population risk.9 When cardiac anomalies are considered in terms of their developmental mechanism rather than by anatomy,14 there seems to be a higher recurrence risk for lesions due to abnormal flow patterns, which includes hypoplastic left heart syndrome, coarctation of the aorta, aortic valve stenosis, pulmonary valve stenosis, and membranous ventricular septal defects.1,2,7,9,13 When CHD does recur, it is not necessarily the same anatomical defect. The concordance rate (i.e., whether recurrences were the same abnormality as in the first affected family member) varies widely. In one study, 37% of cases were reported to have exact concordance, though the rates ranged from 0 to 80% depending on the type of CHD.15 Isolated atrioventricular septal defect (with normal cardiac situs) had a concordance rate of 80%, whereas atrial septal defects had a 0% concordance rate for the same abnormality.15 When considered in broadly defined groups of CHD, the concordance rates are higher than for the exact abnormality recurrence.15 Ventricular septal defects had an exact concordance of 55%, but the nonconcordant cases varied widely and included some severe forms of CHD. Thus a minor form of CHD in the index case does not exclude more severe CHD in recurring cases.15 Also, severe CHD in the index case does not necessarily mean severe CHD when there is recurrence.15 It is of some interest that aortic valvular stenosis seems to be one of the lesions with a higher recurrence risk.1,2 When the mother has aortic stenosis, the recurrence risk to the fetus may be as high as 18%.13 When recurring, an exact concordance rate of 38% has been reported for aortic stenosis.15 Severe aortic valve stenosis is one of the few cardiac structural lesions for which prenatal intervention has been attempted. In utero balloon valvuloplasty has been attempted at 21 to 33 weeks in fetuses with severe aortic stenosis, with the hope of preventing progression to hypoplastic left heart syndrome.16,2 Initial results have been variable. Whether closer scrutiny and earlier diagnosis of fetuses at higher risk for aortic stenosis would contribute to improved outcome is not clear. The association of cardiac anomalies with deletions in the long arm (q) of chromosome 22 has been increasingly recognized over the past several years. It is important to be aware of this particular association because other than trisomy 21, deletions in 22q11 are believed to be the most important chromosomal cause of heart malformations.18 The majority of patients with DiGeorge syndrome and velocardiofacial (Shprintzen’s) syndrome have deletions in chromosome 22q11.18 The clinical features of these two syndromes overlap, but the majority of patients with either syndrome have cardiac defects. Although many types of CHD have been reported in patients with deletions in 22q11, the most common cardiac defects associated with 22q11 deletions include tetralogy of Fallot (TOF), pulmonary atresia, truncus arteriosus, and type B interrupted aortic arch.19–21 Renal abnormalities, which may also be identified prenatally, have been reported in association with 22q11 deletions.20,2 About three fourths of cases of 22q11 deletions arise de novo, and the other one fourth are inherited in an autosomal dominant manner.18,2 Individuals who have a 22q11 deletion have a 50% risk of transmitting the deletion to their offspring.18 The pregnant patient with an increased risk for fetal cardiac anomalies, such as a family history of congenital heart disease, is understandably anxious. The parents’ level of anxiety will likely vary with their past experience. Parents whose child has had serious heart disease with numerous hospitalizations and surgery are likely to be more anxious than those whose child had a minor defect such as a small, isolated ventricular septal defect. With a properly performed obstetric sonogram, one can identify the majority of fetuses with cardiac anomalies. In the following sections, I discuss when and how to perform the sonographic exam, reasonable expectations of sonography for identifying CHD, and the sonographic features of the more common structural cardiac anomalies. At what gestational age should one examine the fetal heart by sonography? It is important to identify CHD as early as possible, but also to perform the exam at a gestational age when the heart can be adequately evaluated. Although the push is generally for an earlier diagnosis, one should realize that, though uncommon, a few cardiac lesions (such as cardiomyopathies, some cases of valvular stenosis, and tumors) may not develop until later in gestation.23 Not surprisingly, the ability to obtain the four-chamber (4C) view in normal second-trimester fetuses increases with gestational age. In a prior study of fetuses at 16 to 17 weeks gestational age, the 4C view was obtained in ~80% of normal fetuses, increasing to 95% or greater after 18 weeks.22 Adequate views of the aortic and pulmonary outflow tracts were also obtained in more than 95% of fetuses after 18 weeks.24–26 In general, the fetal heart tends to be best seen between ~20 and 30 weeks. It has been suggested that in a low-risk pregnancy, around 25 to 30 weeks would probably be the ideal time to scan if one just considers the goals of obtaining the best ultrasound images and not missing late-developing lesions.23 However, one would generally want to scan earlier if termination of pregnancy is an option, and around 20 weeks would probably be a reasonable time in a low-risk pregnancy.23 However, many so-called routine second-trimester obstetric sonograms in the United States are performed at around 16 to 18 weeks, and the fetal heart can usually be adequately evaluated at that time. For the fetus with an increased risk of CHD, a targeted evaluation of the heart would traditionally be done around 18 to 22 weeks gestational age. There will be a small minority of patients at increased risk for fetal CHD who will need follow-up sonograms due to an inability to fully evaluate the heart for such technical reasons as maternal obesity or poor fetal position. There is another option for earlier diagnosis in some centers now. Evaluation of the fetal heart, using both transabdominal and transvaginal transducers, can be attempted at around 12 to 14 weeks. The expertise to perform such exams is not available at all centers, but will probably become more widely available with time. The majority of anomalies diagnosed at this earlier gestational age are severe, complex anomalies.27 With transvaginal sonography the 4C and outflow tract views can be obtained in 70 to 100% of fetuses at 13 to 14 weeks gestational age.27 Transvaginal sonography is limited by restricted angles of imaging, decreased penetration, and the small size of the fetal heart.27 Not surprisingly, a complete exam is more likely to be obtained closer to 14 weeks than at 12 weeks. In experienced hands, this early scanning in the late first trimester seems to be able to identify, or at least suspect, the majority of CHD.28,2 A normal scan at 12 to 14 weeks can offer preliminary reassurance to the patient, but should be followed up with another ultrasound later in pregnancy, probably around 20 to 22 weeks.23 A wide range (4 to 96%) has been reported for the sensitivity of the 4C view for identifying CHD in the second trimester.5 Although several factors may help explain the variable sensitivity in different studies, a large part of the variable sensitivity probably has to do with how well the heart is seen and what criteria are used to consider the 4C view as normal. There is more to the 4C view than just seeing four chambers. In the next section, what constitutes an adequate 4C view is discussed. In the Routine Antenatal Diagnostic Imaging Ultrasound Study (RADIUS), the sensitivity for “complex” CHD using the 4C view was 43%.30 A reasonable estimate of the sensitivity of the 4C view for CHD is probably around 50 to 60%, increasing to about 80 to 85% when the outflow tracts are also evaluated.25,31,32 The variably reported sensitivity, however, should reinforce the notion that more consistent and complete sonographic examinations are needed to achieve optimal sensitivity.5 There are several cardiac anomalies that are particularly difficult to detect even with an optimal sonographic exam. These include patent ductus arteriosus (PDA), secundum atrial septal defect (ASD), small ventricular septal defect (VSD), mild degrees of aortic or pulmonary valve stenosis, anomalous pulmonary venous return, and coarctation of the aorta. PDA and secundum ASD (the most frequent type of ASD) are rarely, if ever, diagnosed prenatally because the ductus arteriosus and foramen ovale are normally patent in the fetus. Small VSDs, mild degrees of aortic or pulmonary valve stenoses, anomalous pulmonary venous return, and coarctation of the aorta are sometimes difficult to diagnose because the lesion is small or produces only minor changes in the appearance of the 4C or outflow tract views. It is important for parents to be aware of these limitations of fetal cardiac sonography. Because ASDs and small VSDs are relatively common lesions, it is particularly important for parents who have a previous child with one of these lesions to understand the limitations of sonography. Although the prenatal diagnosis of a specific cardiac lesion may not always be exactly correct, false-positive diagnoses of CHD are uncommon. Small VSDs and coarctation of the aorta are probably the most frequent false-positive diagnoses and will be discussed subsequently. Different approaches and views have been suggested to evaluate the fetal heart, although the 4C view is the basic view used by most sonographers and sonologists. The 4C view is taken on a transverse view through the fetal thorax (Fig. 16–1). For the 4C view to be considered normal, one should be able to determine that (1) the four chambers are normal in size, (2) the ventricular septum is intact, (3) the “crux” region of the heart is intact, and (4) the general appearance (i.e., size, position, and axis) of the heart is normal.33–35 The two atria are generally of the same width, as are the two ventricles, although the right-sided chambers of the heart may be minimally larger than the left-sided chambers later in gestation. The ventricular septum should be continuous, with no defect in it. The membranous portion of the ventricular septum is normally thin and, as will be discussed subsequently, may be difficult to image well. The “crux” of the heart refers to the area where the ventricular and atrial septa meet the atrioventricular valves.33 At the crux, the septa should be intact, and the tricuspid valve should appear to attach to the septum just slightly more toward the apex of the heart than does the mitral valve. Figure 16–1 Four-chamber (4C) view. The ventricles are approximately equal in size as are the atria in this normal 4C view. The ventricular septum is intact (arrow is within left ventricle and points to ventricular septum). The crux region (curved arrow is within the right atrium and points to the crux where the tricuspid valve is attached) is intact, with the tricuspid valve slightly more toward the apex than the mitral valve at this level. Two of the pulmonary veins (arrow-heads) can be seen entering the left atrium. (Image courtesy of William J. Watson, M.D., Rochester, MN) Overall assessment of the heart reveals that it normally occupies about one third of the cross-sectional area of the thorax. The heart is basically midline, but with the cardiac apex to the left, and the ventricular septum is usually oriented at about a 45-degree angle to the mid-line sagittal plane.36,2 The normal range of the cardiac axis is reported as 20 to 55 degrees in one study37 and 22 to 75 degrees in another study.36 Although fetuses with CHD may have a normal cardiac axis, an abnormal cardiac axis is frequently associated with CHD.36–38 Ideally, one should also determine if the abdominal situs (position of abdominal organs) is normal. The stomach should be on the left side of the fetus, and, particularly if one suspects a situs abnormality, the inferior vena cava should be assessed for abnormal location or for interruption of its hepatic segment, which may occur with polysplenia or asplenia. Figure 16–2 Left ventricular outflow tract views. (A) This view from a normal fetus demonstrates continuity of the ventricular septum (thick arrow within the left ventricle that points to the septum) and the wall of the aorta (thin arrow). A portion of the right ventricle (R) is seen on the other side of the ventricular septum. This view is used to assess for an overriding aorta, and one may not recognize an overriding aorta if the view does not include at least a small portion of the right ventricular lumen. (Reprinted with permission from Brown DL, Hornberger LK. Problems and pitfalls in the sonographic diagnosis of fetal cardiac anomalies. Ultrasound Q 1995;13:221–227.) (B) This view from a different fetus illustrates an inadequate left ventricular outflow tract view. It may be tempting to accept a view like this as normal because it seems to show a normal aorta, but it is not adequate to exclude overriding of the aorta. Although it does show the aorta (thin arrow) arising from the left ventricle, it does not adequately demonstrate continuity of the ventricular septum and aorta (thick arrow is within the left ventricle and points to part of the ventricular septum). Other views showed overriding of the aorta in this fetus who had tetralogy of Fallot. One should be able to see a portion of the right ventricular lumen, as in (A), on the other side of the ventricular septum before continuity of the ventricular septum and aorta can be adequately evaluated. New guidelines for obstetric ultrasound were jointly developed by the American Institute of Ultrasound in Medicine (AIUM), the American College of Radiology (ACR), and the American College of Obstetricians and Gynecologists (ACOG); they were published in 2003.39 The guidelines state that the basic cardiac exam includes a 4C view of the fetal heart. It further states that, if technically feasible, an extended basic cardiac examination can also be attempted to evaluate both outflow tracts.39 Including the right and left ventricular outflow tracts will help identify abnormalities of the great arteries that are likely to be missed on the 4C view alone.26,2 We find a long-axis view most helpful for evaluating the left ventricular outflow tract (Fig. 16–2). It is attained by slight cranial angulation (with respect to the fetus) of the transducer from the 4C view and slight rotation of the transducer toward the fetal right shoulder.6,2 The key feature to note on 4C view is that the ventricular septum is continuous with the anterior wall of the aorta. The right ventricular outflow tract can be imaged with slightly more cranial angulation and rotation of the transducer in the opposite direction. This gives a short-axis view, which shows bifurcation of the main pulmonary artery (PA) into the right PA and ductus arteriosus (Fig. 16–3). The left PA is not seen in this plane. A key feature to note in obtaining the two outflow tract views is that the great arteries cross at right angles to each other as they exit the heart. The PA is usually slightly larger than the aorta.41 One could argue that, although color Doppler is sometimes helpful,42–44 it is not generally needed for evaluation of the normal fetal heart.42 Color Doppler is most helpful when an abnormality is suspected, particularly abnormalities of the great arteries or pulmonary veins, and to assess for valvular regurgitation. Some investigators, however, feel that color Doppler does result in increased detection of cardiac abnormalities, at least in patients at increased risk for trisomy 21.45 From the foregoing discussion, there are six key features (four features on the 4C view and two features on the two outflow tract views) that will allow one to decide if the fetal heart is normal or abnormal. The six features, posed as questions, are listed here. If all six questions can confidently be answered “yes,” then the heart is very likely to be normal. Regarding the 4C view, the following are important: 1. Are four chambers present and symmetric in size? 2. Is the ventricular septum intact? 3. Is the crux region of the heart normal? 4. Are the size, position, and axis of the heart in the chest normal? Figure 16–3
Risk Factors and Hereditary Issues for Congenital Heart Disease
Timing of the Ultrasound Exam of the Fetal Heart
Sensitivity of Ultrasound for Identifying Congenital Heart Disease
Ultrasound Evaluation
General Aspects
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree