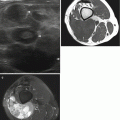
Fig. 13.1
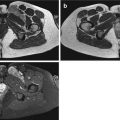
(a–f) Two cases of nodular fasciitis, fascial type: a first case (a–c) at the fascia of the gracilis muscle and a second case (d–f) at the fascia of the pectoralis muscle. (a) Axial spin-echo T1-weighted MR image. (b) Axial spin-echo T1-weighted MR image after gadolinium contrast injection. (c) Axial spin-echo T2-weighted MR image. (d) Sagittal spin-echo T1-weighted MR image. (e) Sagittal spin-echo T1-weighted MR image after gadolinium contrast injection, with fat suppression. (f) Axial spin-echo T2-weighted MR image. Both lesions are in close contact with superficial fasciae. On T1-weighted images, both lesions are of slightly higher signal intensity (SI) when compared with SI of normal muscle (a, d). They both enhance markedly after contrast injection (b, e) and are of high SI on T2-weighted MR images (c, f)
The cause is unknown and for long a trigger by local injury or a local inflammatory process was most likely. More recently, concurrent presentation of nodular fasciitis and aneurysmal bone cyst has been reported, with both tumors belonging to the biologic spectrum of USP6-induced tumors [121, 166].
There are three subtypes of nodular fasciitis according to their topography: the most common subcutaneous type, the intramuscular type, and the fascial type, which spreads along superficial fascial planes. Microscopically, nodular fasciitis can be subdivided into three types based on the predominant histological features: myxoid (type 1), cellular (type 2), and fibrous (type 3). However, since many different features may coexist in one lesion, definition of a single fixed type is not always possible. Microscopically, younger lesions consist of fibroblasts embedded in a dense reticulin meshwork and birefringent collagen. There is a rich intervening myxoid matrix. Older lesions tend to be more fibrous. Histological transformation from type 1 to type 2 and then to type 3 is indicative of the natural evolution of these lesions. Recurrence after excision is very rare and occurs in less than 2 % of the cases.
On ultrasound images, nodular fasciitis appears mostly solid, but cystic change has been seen. Superficial-type nodular fasciitis is reported to have a hypoechoic appearance with echogenic foci or peripheral hyperechoic nodules and quite often does not show internal vascular flow [87]. On CT scans, nodular fasciitis has low attenuation values, reflecting the myxoid character of the lesions [104]. The appearance on MR images reflects the gross morphology of the tumor. Myxoid and cellular lesions are hypointense or iso- to slightly hyperintense compared with skeletal muscle on T1-weighted images and iso- to hyperintense compared with fat on T2-weighted images. Lesions with a more fibrous histology are markedly hypointense on all spin-echo sequences [104]. Myxoid lesions show a marked and homogeneous enhancement, cellular lesions a nonhomogeneous enhancement, and fibrous lesions a moderate, nonhomogeneous enhancement [159]. Three cases in our own series had identical appearance on MR images: a central area of low signal intensity (SI) and a peripheral area of higher SI on T1-weighted images and an inverted SI pattern (high SI at the center and lower SI at the periphery) on T2-weighted images. This pattern was described in 1991 by Frei et al. [46]. On comparing these findings with the MR features of some neurogenic tumors (see Sect. 17.2.2), we named the pattern “the inverted target sign.” After gadolinium (Gd) contrast injection, there was a marked enhancement of the peripheral zone, with poor enhancement of the (myxoid) center (Figs. 13.1 and 13.2). In a study on MRI appearance of 29 lesions, twelve lesions exhibited central nonenhancing areas. Trans-compartmental spread was demonstrated in nine lesions. Osseous changes were seen in five cases and included extrinsic cortical saucerization, medullary edema, and transcortical osseous invasion. Two lesions demonstrated intra-articular extension [16].


Fig. 13.2
(a–g) Three cases of nodular fasciitis, intramuscular type: the first case (a–c) at the forearm, the second case (d, e) at the subscapularis muscle, and the third case (f, g) at the pectoralis muscle. (a) Axial spin-echo T1-weighted MR image. (b) Coronal spin-echo T1-weighted MR image after gadolinium contrast injection. (c) Axial spin-echo T2-weighted MR image. (d) Axial spin-echo T2-weighted MR image. (e) Axial spin-echo T1-weighted MR image after gadolinium contrast injection, with fat suppression. (f) Coronal spin-echo T1-weighted MR image, with fat suppression. (g) Coronal spin-echo T1-weighted MR image after gadolinium contrast injection, with fat suppression. All three cases present with the so-called “inverted target” sign consisting of a peripheral zone of increased SI on T1-weighted MR images (a, f), decreased SI on T2-weighted MR images (c, d), and merely peripheral contrast enhancement (b, e, g)
The cellular type of nodular fasciitis is apt to be mistaken for a sarcoma (myxofibrosarcoma and fibrosarcoma), while the fibrous type must be differentiated from fibromatosis. Recurrent tumors have a more aggressive behavior than primary ones (Fig. 13.3).


Fig. 13.3
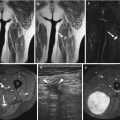
(a, b) Recurrent nodular fasciitis of the fascial type in a 37-year-old woman. (a) Axial spin-echo T1-weighted MR image. (b) Axial spin-echo T2-weighted MR image. Well-delineated, oval mass within the region of the infraspinatus muscle, adjacent to the scapula. The lesion is hyperintense to muscle on the T1-weighted image, with a central, bilobate area of lower signal intensity (a). Central parts of the lesion show high signal intensity on the T2-weighted image (b), while the peripheral component remains of lower signal intensity. This case also illustrates the “inverted target sign”
13.2.2 Proliferative Myositis (and Fasciitis)
Proliferative myositis is a rare pseudosarcomatous process similar to proliferative fasciitis (histologically the subcutaneous counterpart). Lesions present in middle-aged and older adults, arising predominantly in the trunk, shoulder girdle, and upper arm and less often in the thigh. Typically, a painful swelling is noted with subsequent rapid growth (even over days). This tumoral lesion is benign and most often self-limiting with no tendency to recur [33]. On pathology, proliferative myositis demonstrates intense fibroblast proliferation in the stromal tissue (perimysium and epimysium), creating the characteristic “checkerboard” pattern by extending between normal muscle fibers. Microscopically, plump fibroblastic/myofibroblastic spindle cells together with larger basophilic cells resembling ganglion-like cells are seen with normal mitotic figures.
On imaging, proliferative myositis may present with characteristic features that should prompt the radiologist to include this entity in the differential diagnosis with sarcomatous lesions. The solid, ill-defined mass often presents fusiform, demonstrating thickened bands with interspersed continuous muscle fibers on longitudinal images and the “checkerboard” pattern on transverse images. On transverse ultrasound, hypoechoic linear structures are grouping bundles of muscle fibers in a “checkerboard” pattern or if grouping more randomly resembling “dry-cracked mud” (Fig. 13.4) [133]. On duplex ultrasound, vascularization may be normal or increased. CT may show an ill-defined, expansile lesion that is isointense or hypointense compared to muscle. MR imaging demonstrates a focal intramuscular expansion appearing moderately or strongly hyperintense on T2-weighted images and hypointense or isointense on T1-weighted images with marked enhancement after contrast administration. MR imaging demonstrates equally well the “checkerboard” pattern on transverse images and the preserved, continuous muscle fibers within the lesion on longitudinal images (Fig. 13.5). Moreover, MR imaging shows perilesional edema and contrast enhancement extending to the surrounding fascia, suggesting inflammatory changes [168].



Fig. 13.4
Proliferative myositis in a 52-year-old female aerobics instructor with 3-week history of a painful swelling in the anteromedial aspect of the upper arm. (a) Axial T2-weighted MRI view, (b) coronal STIR image, and (c, d) axial contrast-enhanced T1-weighted MR images. (a, b) The mass appears inhomogeneously hyperintense in T2-weighted image (large black arrows). The hypointensities within the lesion have geometrical contours on axial images – consistent with “checkerboard-like pattern” – and are clearly continued with muscle fascicles at the upper and lower pole of the tumor in coronal image (small black arrows). The inferior margin of the lesion cannot be identified and seems to continue within the interfascicular space inferiorly (empty white arrow). (c) Contrast-enhanced T1-weighted MRI view showing marked inhomogeneous enhancement (dashed white arrow) and central branched area of decreased signal intensity tracing the interfascicular spaces (small white arrows), again consistent with “checkerboard-like pattern.” (d) Inferior to the tumor, reactive fasciitis of the biceps brachialis (plain white arrows) and the anterior medial part of the triceps (white arrowheads) is noted with increased signal in STIR and contrast enhancement in T1-weighted image (Reprinted with permission from Jarraya et al. [67])

Fig. 13.5
Follow-up 7 weeks later in the same patient as in Fig. 13.4 (a) axial STIR and (b) axial contrast-enhanced T1-weighted MR image demonstrate complete resolution of the intramuscular mass with persistence of slight fascial STIR hyperintensity and contrast enhancement at the anterior medial aspect of the biceps brachialis (plain arrows) and along the biopsy path (dashed arrow) (Reprinted with permission from Jarraya et al. [67])
13.2.3 Myositis Ossificans and Fibro-osseous Pseudotumor of the Digits
Definition. Myositis ossificans is a generally solitary, benign, self-limiting ossifying process occurring in the musculature of the extremities in young men and is related to trauma in about half of all cases [32, 100]. Sometimes, it occurs within other tissues, such as subcutaneous fat (panniculitis ossificans) – in one-third of the cases – tendons or fasciae (fasciitis ossificans), and periosteum of the digits (fibro-osseous pseudotumor of the digits) [32, 83, 92, 100, 109].
Most lesions in myositis ossificans measure 3–6 cm in diameter. On cross section they have a white, soft, and rather gelatinous center and firm, yellow-gray periphery with rough, granular surface [32]. Microscopically, a zonal pattern is observed. This refers to a progressive degree of cellular maturation from the center to the periphery, maturation being lowest in the center and highest with mature bone formation – at the periphery.
Incidence and Clinical Behavior. Myositis ossificans is by far the most common bone-forming lesion of the soft tissues. The exact pathogenesis of this disorder is still unclear. A history of preceding mechanical trauma is present in about half of all cases. As causative factors in some of the other cases, infection and coagulopathy have been mentioned. Furthermore, the disease may also occur in association with burns, paraplegia, and quadriplegia or with other neuromuscular disorders such as tetanus [32, 100].
Myositis ossificans commonly affects young, active adults and adolescents, predominantly men (Figs. 13.6, 13.7, 13.8, and 13.9), but occasionally involves persons of other age groups. Pain and tenderness are the first symptoms, followed by a diffuse swelling of soft tissues. This swelling typically becomes more circumscribed and indurated after 2–3 weeks. Thereafter, it progressively changes into a firm hard mass approximately 3–6 cm in diameter, which is well outlined on palpation. Although malignant transformation into extraskeletal osteosarcoma has been suggested in the literature, it has never been proven. Hence, the prognosis of myositis ossificans is generally accepted to be excellent [32].





Fig. 13.6
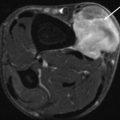
(a–c) Myositis ossificans, early stage, in the left upper arm of a 7-year-old boy: (a) coronal T1-weighted MR image, (b) coronal T1-weighted MR image after gadolinium contrast injection, and (c) coronal T2-weighted MR image. Ill-defined mass within the biceps muscle. On the T1-weighted image, the mass is slightly hyperintense to the muscle (a). After contrast injection there is a marked peripheral enhancement (b). On the T2-weighted image, the center of the lesion remains hypointense, while the hyperintense periphery is outlined by a thin hypointense rim. Extremely high signal intensity within the whole biceps muscle (c). Characteristic appearance of an early-stage myositis ossificans (biopsy revealed large amounts of osteoid bone, surrounded by numerous osteoblasts)

Fig. 13.7
(a–c) Myositis ossificans, intermediate stage of the right thigh in a 13-year-old boy: (a) plain radiograph a few days after trauma, (b) plain radiograph 5 days later, and (c) CT. Presence of a faint linear periosteal calcification on the ventral aspect of the right thigh (a). Five days later there is extensive calcification within the quadriceps muscle (b). On CT there is diffuse swelling of the right thigh with an eggshell calcification within the vastus intermedius muscle (c). The zoning phenomenon is characteristic for an intermediate stage of posttraumatic myositis ossificans

Fig. 13.8
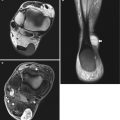
(a–f) Myositis ossificans, mature stage, in the thigh of a 19-year-old professional female rower: (a) plain radiograph, (b) CT, (c) scintigraphy, (d) coronal spin-echo T1-weighted MR image, (e) axial spin-echo T1-weighted MR image after gadolinium contrast injection, and (f) axial spin-echo T2-weighted MR image. The plain radiograph reveals a rounded calcified soft tissue mass posteriorly in the thigh (a). CT confirms the presence of a heavily calcified lesion within a slightly hypotrophic gluteus maximus muscle. A small central zone remains uncalcified (b). On the radionuclide scan, there is an intense tracer fixation posteriorly in the left trochanteric region (c). The lesion is hypointense on the T1-weighted image (d), is hyperintense on the T2-weighted image (f), and shows marked enhancement (onionskin pattern) after contrast injection (e). Absence of concomitant mass effect is also obvious on all MR images. History of the patient and imaging findings are in favor of a mature myositis ossificans. Despite the huge calcifications, the lesion still generates high signal intensity on the T2-weighted image

Fig. 13.9
(a, b) Posttraumatic myositis ossificans, mature stage, anteriorly in the proximal third of both thighs in a 33-year-old man: (a) CT at the level of the lesser trochanter of the femur (soft tissue window setting); (b) same CT section as in a (bone window setting). Huge, considerably calcified soft tissue masses within both quadriceps muscles, corresponding to the mature stage of myositis ossificans
Principal sites of involvement are the limbs, which are affected in more than 80 % of cases. The quadriceps muscle and brachialis muscle are favored sites in the lower and upper extremity, respectively. Areas prone to trauma are more commonly afflicted.
The incidence of panniculitis ossificans differs slightly from that of myositis ossificans in that it prevails in the upper extremities of women [32, 100].
Fibro-osseous pseudotumor of the digits occurs predominantly in the fingers or toes of young adults, more often in females [83].
Imaging. Acute myositis ossificans refers to early stages of disease, before ossification is radiologically visible. During these initial stages of the disease, only a slight increase in soft tissue density is observed radiologically. In general, calcification develops between 4 and 6 weeks after the initial trauma and results in a “mature” lesion. Initially, these calcifications present as irregular, floccular opacities on radiography. Over time the lamellar bone forms at the periphery of the lesion and proceeds toward its center [32, 100]. The centrifugal pattern of progressive maturation is well reflected by the CT appearance of the lesion during the active stage of disease: the central immature zone of the lesion appears radiolucent, whereas the outer mature zone shows calcification and ossification (Figs. 13.7 and 13.8). This appearance is referred to as the “zoning” phenomenon.
Three different appearances of myositis ossificans are noted on MRI, corresponding to the stage of maturation. Early stages of myositis ossificans, the so-called acute form, present on MRI as a mass that is isointense or even slightly hyperintense to muscle on T1-weighted images, but hyperintense on T2-weighted images (Fig. 13.6). The lesion is surrounded with variable amounts of edema, appearing hyperintense on T2-weighted image and with a hypointense rim in some cases. Following administration of gadolinium, a well-defined rim of enhancement is observed, allowing differentiation between the lesion and primary soft tissue sarcoma, which is enhanced homogeneously. The MRI appearance of the lesions during the intermediate or subacute stage is characterized by isointensity with muscle on T1-weighted images and mild increase of signal intensity on T2-weighted images [153]. These findings are explained by a central fibrous transformation as observed histologically. Occasionally, a thin rim of signal void surrounding the lesion may be observed, especially on T2-weighted images, and corresponds to a rim of calcification, although this is better observed on plain radiography and CT. Findings consistent with hemorrhage and fluid-fluid levels are rarely seen. Mature lesions (i.e., the “chronic stage”) show more extensive signal voids on all sequences, corresponding to a considerable degree of peripheral calcification and ossification. In this stage lesions demonstrate increased signal intensity in an “onionskin pattern” on T2-weighted images (Fig. 13.8).
The diagnosis of myositis ossificans commonly relies on findings on plain radiography. Attention must be paid to the presence of a central radiolucent area, as a manifestation of the zoning phenomenon and of a lucent line separating the lesion from the underlying cortex, which are both better demonstrated on CT. As biopsies, establishing the diagnosis, may have been taken during early stages of the disease, the lesion may continue to grow for some period of time. In these cases repeated plain radiographs and CT are useful to document the maturation and to exclude a destructive growth pattern (Fig. 13.9) [84]. Plain radiography and CT are superior to MRI in demonstrating calcifications and ossification; however, in the case of early disease – “acute myositis ossificans” – MRI has proven the most accurate imaging technique, although findings are nonspecific.
The appearance of panniculitis ossificans and fibro-osseous pseudotumor of the digits is similar to that of myositis ossificans except that these conditions may lack an obvious zoning phenomenon. In the latter case, cortical erosion of the underlying bone and stippled calcification may be observed (Fig. 13.10) [83, 92, 100, 109].


Fig. 13.10
(a–d) Fibro-osseous pseudotumor of the digit. (a, b) Hypothenar eminence swelling with a red area in the skin. Note the small punctual scar lesion due to core needle biopsy of the fifth metacarpal bone. (c) Anteroposterior X-ray demonstrated a calcified mass in soft tissues close to the periosteal surface. (d) Evolution (shrinkage) of the lesion 6 months later was useful to make an accurate diagnosis of fibro-osseous pseudotumor of the digit (Reprinted with permission from Liliana and Santini-Araujo [92])
13.2.4 Ischemic Fasciitis
Ischemic fasciitis, also called decubital fibroplasia or decubital fasciitis, is a pseudosarcomatous lesion presenting as a mass in areas overlying bony prominences. These lesions occur predominantly in elderly patients and can be associated with physical immobility. Usually the limb girdles, sacrum, greater trochanter, shoulders, buttocks, or posterior chest wall are involved. Histologically, a zonal appearance is noted with pseudosarcomatous proliferation of (myo-) fibroblasts and ganglion-like cells with a central area of fibrinoid degeneration that may include infarcted fat surrounded by granulation tissue (the latter distinguishing it from proliferative and nodular fasciitis).
MRI signal intensities are nonspecific with low signal on T1- and high signal on T2-weighted images. After intravenous contrast administration, an irregular area of central hypointensity can be seen within the enhancing mass, possibly corresponding to the central area of fibrinoid degeneration/infarcted fat (Fig. 13.11) [64]. Perilesional edema may be seen extending beyond the fascia. Features that may help to differentiate from necrotic sarcoma and abscess include the subcutaneous location at a pressure point in an elderly, immobile patient. Surgical debridement or resection sometimes with rearrangement of local soft tissue is the treatment of choice, and recurrences are rare [134] [11].


Fig. 13.11
Ischemic fasciitis, also called decubital fasciitis. A 77-year-old immobilized woman presenting with a hard, palpable, and fixed mass over the greater trochanter. MR imaging revealed a subcutaneous mass lesion abutting the iliotibial band. (a) On T1-weighted images (WI), the lesion was isointense to the muscle (white arrow), with a central focus of high signal intensity. (b) Fat-suppressed (FS) T2-WI showed predominantly hyperintense signal (black arrowheads) with intralesional foci of low SI, adjacent to the right greater trochanter (white asterisk). (c) After intravenous administration of gadolinium contrast, a marked enhancement was seen with small areas of nonenhancement, in keeping with necrotic foci (white arrow). Neither invasion of the gluteus muscles nor the bone marrow of the greater trochanter was seen. Histological examination revealed the diagnosis of ischemic fasciitis, with a central hypocellular area surrounded by a fibroblastic and vascular proliferating outer zone (Reproduced with permission from Ceulemans L, et al. [92])
13.2.5 Elastofibroma
Elastofibroma is generally considered to be a fibroelastic pseudotumor. It occurs almost exclusively in the subscapular region deep to the rhomboid major and latissimus dorsi muscles adjacent to the inferior angle of the scapula, in close relation with the serratus anterior muscle and ribs. It is thought to be caused by repeated mechanical friction between the chest wall and the tip of the scapula, and this hypothesis is supported by immunohistochemical data [25]. Other locations, albeit uncommon, include the infraolecranon, thoracic wall, axilla, and greater trochanter regions. Bilateral lesions are common and seen in 10–60 % of patients. Most patients are older adults, and there is a definite predominance in women. Single cases have been reported in children and in an infant [8, 24]. Half of the patients are asymptomatic, the other half presenting with pain or restricted upper limb motion [72]. Chromosomal abnormalities and familial occurrences support a genetic predisposition to elastofibroma dorsi.
On gross examination, these lesions are firm and rubbery [24, 82, 91, 99]. Histologically, lesions are composed mainly of elastin-like fibers and entrapped islands of mature adipose tissue. Microscopic examination shows hypertrophy and degeneration of elastin with a background of mature collagen and fat. CT scans show attenuation similar to that of adjacent muscle. Sometimes, there are strands of low density similar to that of subcutaneous fat [85] (Fig. 13.12a). Lesion size on CT correlates best with the size of the postoperative pathologic specimen, compared to US and MRI [107]. MRI nicely reflects the histological composition of entrapped fat within a predominantly fibrous mass. On both T1- and T2-weighted images, the lesion presents as a lenticular, well-defined mass with an intermediate SI approximately equal to that of skeletal muscle. Interlaced areas have a SI similar to that of fat [81, 97]. Areas of low SI on both T1- and T2-weighted images correspond histologically to fibroelastic tissue [85] (Fig. 13.12b–d). Some lesions may present with mild to moderate uptake on 18F-FDG PET and/or diffusion restriction on MRI and should not be mistaken for metastasis [35, 60].


Fig. 13.12
(a–d) Elastofibroma dorsi in a 60-year-old woman (a), in a 67-year-old woman (b, c), and in a 56-year-old woman (d). A CT scan after iodinated contrast injection. (b) Coronal spin-echo T1-weighted MR image. (c) Axial turbo spin-echo T2-weighted MR image. (d) Coronal STIR MR image. On the CT scan, there is an oval mass between the rhomboid major and latissimus dorsi muscles on both sides (arrows). The lesions show mixed attenuation and enhancement after iodinated contrast injection (a). Oval mass within the subscapular region, showing signal intensity equal to that of fat and muscle on a T1-weighted image (b), and an overall low signal intensity on T2-weighted on STIR images (c, d). Three cases of elastofibroma showing characteristic location, attenuation on CT scan, and signal intensity on MR images, reflecting the histological composition of entrapped fat within a fibrous mass
Surgery should not be performed for diagnostic purposes only in elderly individuals and should be reserved for symptomatic lesions [12]. Indeed, familiarity of the radiologist with this entity may make it possible to avoid some surgery [98]. Differential diagnosis is limited, but includes older desmoids, which are hypocellular, contain large amounts of collagen, and occur in younger patients.
13.2.6 Fibroma of Tendon Sheath
Fibroma of the tendon sheath consists of a benign, slow-growing, dense fibrous nodule (or multinodular mass) firmly attached to the tendon sheath and is found most frequently in the hands and feet (Fig. 13.13). The thumb, index finger, and middle finger together with lesions of the volar aspect of the wrist account for 80 % of cases. It is found most commonly in adults and is more than twice as common in men as in women. The main symptom is a small mass that rarely measures more than 2 cm and is painless but may limit motion of the involved digit [95]. Macroscopically, it resembles the lobular configuration of the much more common giant cell tumor of the tendon sheath, but it is less cellular and there are no xanthoma cells or giant cells [32]. Microscopically, this lobulated nodular lesion is composed of tightly packed spindle cells (fibroblasts), vessels, and large amounts of dense collagenous material, which is markedly hyalinized. Occasionally, there is a gradual transition from the poorly cellular hyalinized collagenous areas to more cellular areas which are thought to be characteristic of the early stages of the tumor development. More cellular forms must be differentiated from nodular fasciitis, fibrous histiocytoma, and even fibrosarcoma.


Fig. 13.13
(a–d) Fibroma of the tendon sheath in a 35-year-old woman. (a) Sagittal spin-echo T1-weighted MR image. (b) Sagittal spin-echo T1-weighted MR image after gadolinium contrast injection. (c) Axial spin-echo T2-weighted MR image. (d) Coronal T2*-weighted image. Large, oval mass at the plantar aspect of the left great toe. The lesion is inhomogeneous and ill-defined at the proximal pole (a). After contrast injection there is marked enhancement at the periphery of the lesion (b). On the T2-weighted image, the whole lesion is of low signal intensity (c). On gradient echo sequence, the periphery of the lesion is of extremely high signal intensity This gradient echo sequence may help to distinguish fibroma from giant cell tumor of the tendon sheath because in the latter an overall low signal is expected due to the T2* effect of hemosiderin (d)
Although fibromas of the tendon sheath may resemble nodular fasciitis histologically, they involve the hands and feet almost exclusively and are slow-growing lesions, while lesions of nodular fasciitis appear suddenly, grow rapidly, and usually attain full size in 3–6 weeks. Moreover, they have a predilection for the volar aspect of the forearm [126]. Recently, intra-articular occurrence of fibroma of the tendon sheath has been reported in the joints such as the knee and the acromioclavicular and temporomandibular joint [53, 56].
Plain radiographic findings in a fibroma of the tendon sheath eroding the third metatarsal have been reported by Lourie et al. [95].
Fox et al. [44] have reported on a series of six patients with fibroma of the tendon sheath. Findings from MRI were not at all specific, i.e., low to intermediate SI on both T1- and T2-weighted images and a variable enhancement (from none to markedly enhanced) after contrast administration. MR findings depend on and reflect the relative amount of different tumor components that are cellular, myxoid, vascular, or collagenous. As a consequence, there can be an overlap in MR findings with features of giant cell tumor of the tendon sheath (Fig. 13.14). Features that may help to differentiate fibroma of the tendon sheath from the latter include the presence of band-like areas of low signal intensity representing collagenous strands, less pronounced contrast enhancement, and lack of significant decrease of signal intensity on gradient echo images (Fig. 13.13d) [20].


Fig. 13.14
(a–c) Fibroma of the tendon sheath at the volar aspect of the index finger adjacent to the flexor tendon. (a) Axial spin-echo T1-weighted MR image. (b) Axial spin-echo T1-weighted MR image after gadolinium contrast injection with fat suppression. (c) Axial spin-echo T2-weighted MR image with fat suppression. The lesion is of low SI on T2-weighted MR images (c) and enhances moderately after gadolinium contrast injection (b)
13.2.7 Nuchal-Type Fibroma
Nuchal-type fibroma, or collagenosis nuchae, is a benign soft tissue tumor that arises from the posterior cervical subcutaneous tissue, with a predilection for the interscapular and paraspinal regions. Nuchal fibroma is significantly more common in men, with a peak incidence during the third to the fifth decades.
Microscopically, nuchal fibromas have a superficial (subcutaneous or dermal) component and consist of paucicellular, thick bundles of lobulated collagen fibers with inconspicuous fibroblasts. Entrapped adipose tissue and traumatic neuroma-like nerve proliferations are typically present. Skeletal muscle infiltration is also seen in a minority of cases. The process has a strong association with diabetes and also appears to be linked to Gardner’s syndrome, fibromatosis, and dermatofibrosarcoma protuberans. Local recurrence probably reflects the persistence of local or systemic factors related to its pathogenesis [105, 131].
The MR appearance reflects the histological composition: collagen has a low SI both on T1- and T2-weighted images. Variable degree of contrast enhancement is reported. Differential diagnosis includes other soft tissue masses with low signal intensity on T2-weighted images and variable enhancement such as desmoid tumor, solitary fibrous tumor, fibroma of the tendon sheath, collagenous fibroma, neurofibroma, cicatricial fibroma, fibrosarcoma, and malignant fibrous histiocytoma [86]. Small foci of high SI on T1-weighted images are due to entrapped adipose tissue (Figs. 13.15 and 13.16). In this regard there is a strong similarity to elastofibromas.



Fig. 13.15
(a–c) Nuchal fibroma in a 50-year-old man. (a) Sagittal spin-echo T1-weighted MR image. (b) Sagittal spin-echo T2-weighted MR image. (c) Sagittal spin-echo T1-weighted MR image after gadolinium contrast injection. Oval, well-delineated mass within the subcutaneous fatty tissue of the neck. There is an intermediate signal intensity on both spin-echo images owing to the mixed histological composition of entrapped fat within a collagenous mass (a, b). There is marked enhancement after contrast injection (c). Localization and MR presentation are characteristic of a nuchal fibroma

Fig. 13.16
(a–c) Nuchal fibroma. (a) Axial spin-echo T1-weighted MR image. (b) Axial spin-echo T1-weighted MR image after gadolinium contrast injection. (c) Axial short-tau inversion recovery (STIR) MR image. Atypical presentation of a nuchal fibroma with high SI on STIR sequence (c) and minimal, cloudy enhancement after contrast injection (b)
13.2.8 Desmoplastic Fibroblastoma (Collagenous Fibroma)
Desmoplastic fibroblastoma or collagenous fibroma commonly occurs in patients in the fifth and sixth decades of life, more frequent in men (75 %). The tumor often manifests as a sharply demarcated, mobile, firm, solitary mass involving the subcutaneous tissue or skeletal muscle. The common locations of the tumor are the arm, shoulder, and posterior neck or upper back. The size of reported collagenous fibromas ranges from l to 20 cm in the largest dimension. Histologically, the tumors appear well marginated on low-power examination. The tumor cells are relatively bland stellate- and spindle-shaped fibroblastic cells separated by densely fibrous to fibromyxoid matrix. The cellularity is low or very low, mitotic figures are very rare or absent, and tumor necrosis is not seen.
On MR images, collagenous fibroma shows areas of decreased SI on T1- and T2-weighted images and moderate to strong contrast enhancement in a heterogeneous fashion [70, 103, 165]. Because both have a high collagen content, similar MRI findings have been described in desmoid tumors (Fig. 13.17). A series of three cases has been reported with a characteristic rim enhancement thought to represent the outer capsule-like fibrous tissue of this usually noninvasive tumor [165]. Collagenous fibromas are less cellular, less vascular, and less infiltrative at their periphery and as a consequence more mobile, better circumscribed, and easier to remove than desmoids. Moreover, they occur in older (more than 40 years old) patients. Differential diagnosis is essential to prevent overtreatment in collagenous fibroma [119].


Fig. 13.17
(a–c) Desmoplastic fibroblastoma. A 53-year-old man with collagenous fibroma of the right knee. (a) Axial T2-weighted image shows low to intermediate signal intensity of a clearly margined mass (white arrows). (b) Sagittal T1-weighted image appears isointense to the muscle (white arrows). (c) Sagittal post-contrast T1-weighted image shows mild internal enhancement with rim enhancement (white arrows). Morphology and MR presentation do not allow differentiation between collagenous fibroma and extra-abdominal desmoids (Reprinted with permission from Yamamoto et al. [165])
13.2.9 Calcifying Aponeurotic Fibroma
Calcifying aponeurotic fibroma is a rare, benign fibroblastic tumor usually occurring in young children and adolescents (therefore also named juvenile aponeurotic fibroma) with a male to female ratio about 2:1. It presents as a unique, hard and painless palpable mass, sized 1–3 cm, with a predilection for the volar side of the hands/fingers and feet [13]. Calcifying aponeurotic fibromas are often associated with tendons and aponeuroses and typically infiltrate the surrounding fatty, muscular, or neurovascular tissues. Histologically, a fibromatosis-like lesion with nodules of calcification is displayed on a background of hyalinized to focally chondroid stroma [37]. In the initial phase of the tumor, which is seen more often in young patients, the tumor shows infiltrative growth and often lacks calcifications, whereas in the late phase, the tumor becomes more compact and nodular, with more prominent cartilaginous differentiation and calcifications [115].
Radiography may sometimes reveal faint, stippled calcification in a focal area of soft tissue swelling, typically in the distal extremities (Fig 13.18) [111]. MR imaging is the best modality to show the extent and margin of the lesion. Signal intensity on T2-weighted images is described as a heterogeneous mixture of high and low signal and seems to be influenced by the degree of calcification and fibrous component: lower T2 signal intensities are more often seen with older age. Signal intensity on T1-weighted images is intermediate. Lesions strongly enhance after contrast medium injection allowing for delineation of the often invasive margins. Differential diagnosis includes tenosynovial giant cell tumor (showing lower signal intensities on T1- and T2-weighted images due to hemosiderin deposition), other fibromatoses, soft tissue chondroma, synovial sarcoma, and clear cell sarcoma [13]. Surgical resection is the preferred treatment. However, the propensity to invade surrounding tissues is reflected in incomplete surgical resection and (late) recurrences up to 50 %.


Fig. 13.18
(a–d) Calcifying aponeurotic fibroma. A 10-year-old girl presented with a firm, elastic, hard mass at the plantar side of the foot. (a) Radiographs revealed faint calcification in the mass (arrowheads) (b) T1-weighted MR image revealed an isointense lesion adherent to the flexor tendon and invading the interdigital webspace. Most of the border appeared well-defined in this case (unusual). (c) On T2-weighted images, there is a heterogeneous mixture of high- and low-signal intensity areas (d) Post-contrast T1-weighted images showed significant enhancement throughout the lesion (Reprinted with permission from Morii et al. [111])
13.3 Fibromatoses
Fibromatoses cover a wide range of benign fibroblastic proliferations. They are classified according to Enzinger and Weiss into superficial and deep types and are further subdivided according to anatomical location:
- 1.
Superficial (fascial) fibromatosis
- (a)
Palmar fibromatosis (Dupuytren’s con-tracture)
- (b)
Plantar fibromatosis (Ledderhose’s dis-ease)
- (c)
Penile fibromatosis (Peyronie disease)
- (d)
Knuckle pads
- (a)
- 2.
Deep (musculoaponeurotic) fibromatosis
- (a)
Extra-abdominal fibromatosis
- (b)
Abdominal fibromatosis
- (c)
Intra-abdominal fibromatosis:
Pelvic fibromatosis
Mesenteric fibromatosis
Gardner’s syndrome
- (a)
Although both superficial and deep lesions have a similar microscopic appearance, superficial lesions are slow growing, while deep ones have a more aggressive biological behavior, between that of fibrosarcomas and fibromas [15]. Division into abdominal and extra-abdominal desmoids is arbitrary, since the lesions are histologically alike [10].
Fibromatoses are uncommon lesions, constituting 0.03 % of all neoplasms. They are found in young adults, with a peak incidence at 30 years. There is no sex predominance, and most lesions are solitary, although synchronous multicentric lesions have been reported. A familial tendency has been observed. Local recurrence after surgery is seen in as many as 50 % of patients [81].
Key criteria that establish the diagnosis are growth pattern, relationship with surrounding tissues, and cytological features. Lesions consist of uniform fibroblasts proliferating in a parallel way and intermingled with variable amounts of collagen. No atypical or hyperchromatic nuclei are found, while mitoses are infrequent. Generally, the most cellular areas are present in the center of the lesion, whereas the periphery has less numerous fibroblasts and greater amounts of dense collagen [45]. Although fibromatoses are histologically benign, their relationship to adjacent tissue is marked by interdigitating and infiltrative growth. Penile fibromatosis and intra-abdominal fibromatosis will not be discussed, since these do not belong to the locomotor system [127].
On ultrasound scans, fibromatoses appear as masses with low, medium, or high echogenicity and smooth, sharply defined borders. On CT images, the masses are either ill-defined or well circumscribed. Before contrast administration, desmoids show variable attenuation. After contrast administration, lesions usually have a higher attenuation than that of adjacent muscle [10].
In an attempt to explain the variable CT appearance of these lesions, Francis et al. have carried out a retrospective analysis of CT findings and histopathological features in nine patients with fibromatosis. In three of four patients who had precontrast CT scans, the tumors were hyperdense relative to the muscle, while in one patient, the lesion was hypodense. The postenhancement appearance was variable. The pathological specimens have been analyzed and graded for collagen content, cellular content, tumor necrosis, and tumor vascularity. No consistent relationship can be established between the CT appearance of these lesions and their histological appearance [45].
On MR images, most lesions demonstrate slightly increased SI relative to the skeletal muscle on T1-weighted images and intermediate SI on T2-weighted images. The slight increase in SI on T1-weighted images is much less than that seen in lipomas and subacute hemorrhages. Enhancement pattern after contrast administration is variable [113, 157].
Increased SI on T2-weighted images is seen in hypercellular lesions. Lesions that are hypointense or contain hypointense foci (linear or curvilinear) on both spin-echo sequences are found to be relatively hypocellular with abundant collagen. As a consequence, most lesions are rather inhomogeneous. Although usually well demarcated on MR images, microscopically fibromatosis is seen to invade adjacent structures [127].
The conditions that deserve consideration in the differential diagnosis of intra- and extra-abdominal desmoids are malignant lesions such as fibrosarcoma, rhabdomyosarcoma, synoviosarcoma, liposarcoma, myxofibrosarcoma, lymphoma, and metastases and benign lesions such as neurofibroma, neuroma and leiomyoma, and acute hematoma of the rectus sheath and chest wall [10].
13.3.1 Palmar Fibromatosis
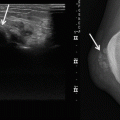
Palmar fibromatosis or Dupuytren’s contracture primarily involves palmar aponeurosis of the hand and its extensions. Usually the disease is diagnosed clinically on the basis of characteristic history and physical examination. The earliest clinical manifestation is the appearance of a subcutaneous nodule in the palm of the hand, most frequently over the fourth and fifth ray. As the disorder progresses, the overlying skin thickens and retracts, and a cord forms, producing progressive flexion contracture of the affected ray (Fig. 13.19).


Fig. 13.19
(a–c) Palmar fibromatosis. (a) Longitudinal Doppler ultrasound. Hypoechoic nodule adjacent to the flexor tendon of the palm of the hand (third ray). (b) Axial SE T1-WI in another patient shows a hypointense lesion at the palmar aspect of the thumb (arrow). (c) Axial FS SE T1-WI after intravenous administration of gadolinium contrast medium shows diffuse enhancement (arrow) (Reprinted with permission from Vanhoenacker et al. [153])
Palmar fibromatosis tends to affect adults, with a rapid increase in incidence with advancing age. It is bilateral in 40–60 % of cases. The condition is by far more frequent in men and is most common in Northern Europe. Pathogenesis in both palmar and plantar fibromatoses includes a genetic component, with family history and chromosome aberrations in 50 % of cases.
Histologically, the nodules are quite cellular, composed of whorls of proliferative myofibroblasts. Cords contain a large amount of collagen and are hypocellular. The condition is treated surgically, but the recurrence rate is high (30–40 %). The recurrence rate of lesions with mitotically active, cellular nodules is far higher (70 %) than that of hypocellular lesions.
The appearance of the nodules and cords on MR images and the correlation between the SI and the lesion’s degree of cellularity have been described by Yacoe et al. [164]. On MR images, the cords had a uniformly low SI (similar to the SI of the tendon) on both T1- and T2-weighted images. Most nodules had an intermediate SI (similar to that of the muscle) on both T1- and T2-weighted images. Some nodules had a low to intermediate SI on T1-weighted images (slightly higher than that of the tendon) and a low SI on T2-weighted images. Few nodules had a low SI on both T1- and T2-weighted images and were hypocellular on histological examination.
Signal characteristics of the lesions correlate with the degree of cellularity. The preoperative assessment of cellularity may be of prognostic significance, because highly cellular lesions tend to have a higher rate of recurrence after surgery than hypocellular lesions. As a consequence surgery may be delayed until the lesion matures and becomes more hypocellular and collagenous [44].
13.3.2 Plantar Fibromatosis
Plantar fibromatosis, also called Ledderhose’s disease, is a benign, fibroblastic, proliferative, and locally invasive disorder characterized by the replacement of elements of the plantar aponeurosis by abnormal fibrous tissue, which slowly invades the skin and the deep structures (Fig. 13.20).


Fig. 13.20
(a–f) Two cases of plantar fibromatosis in a 37-year-old-man (a–c) and in a 13-year-old girl (d–f). (a) Axial spin-echo T1-weighted MR image. (b) Axial turbo spin-echo T2-weighted MR image. (c) Sagittal STIR MR images. (d) Sagittal spin-echo T1-weighted MR image. (e) Sagittal spin-echo T1-weighted MR image after gadolinium contrast injection. (f) Sagittal subtraction MR image. Subcutaneous nodular lesion of intermediate signal intensity at the sole of the foot in apposition with the plantar aponeurosis. The lesion is isointense to the muscle on the T1-weighted image (a) and of higher variable signal intensity on the T2-weighted (b) and STIR (c) images. On the STIR image, there is a smaller second lesion having similar signal characteristics (c; arrow). The second case reveals a lesion centered on the plantar fascia, isointense to the muscle on the T1-weighted image (d), with marked enhancement after gadolinium contrast injection (e). This is also nicely demonstrated on the subtraction image (f)
The plantar aponeurosis is a strong band which originates at the inner tubercle of the calcaneus and contains two layers: a superficial layer which extends to the four smaller toes and a deeper layer which runs from lateral to medial and appears as a separate band to the great toe [89]. On ultrasound scans, the plantar aponeurosis is noted as a bright linear interface, approximately 5–8 mm from the surface of the sole [128]. On MR images the aponeurosis is seen as a thin linear strip of low SI that originates at the inferior border of the calcaneus and inserts into the distal flexor tendons near the metatarsophalangeal joints.
The etiology of plantar fibromatosis is unknown, but, as in palmar fibromatosis, trauma, neuropathy, faulty development, alcoholism, and infection have been proposed as etiologic factors. Pathogenesis in both palmar and plantar fibromatoses includes a genetic component, with family history and chromosome aberrations in 50 % of the cases. Most lesions are located at the medial aspect and just superficial to the aponeurosis. Involvement is bilateral in 19 % of patients and multiple in 32 %. There is a higher prevalence in white and middle-aged (fourth decade) male patients and in patients who have epilepsy. There is also an association with knuckle pads in 5 %, with palmar fibromatosis in 10–50 %, and with penile fibromatosis (Peyronie disease) in 5–10 % of patients. There are no reports of malignant transformation. Clinically, plantar fibromatosis presents as a nonmobile, irregular, single, or multinodular lesion located in the longitudinal medial arch of the plantar surface of the foot. The lesion frequently consists of small nodules merged with each other and with the fascial bundles [5]. Although some authors report pain as a main symptom in one-third of patients, most lesions are asymptomatic and are discovered incidentally by palpation. Unlike palmar fibromatosis, this condition rarely causes contracture of the toes.
Microscopically, the lesion consists of a proliferation of well-differentiated hyperplastic, fibroblast-like cells or myofibroblasts with an infiltrative pattern of growth and usually an abundance of proliferating cells between the heavy strands of relatively hypocellular, mature collagen. Collagen is present in the actively growing foci in every specimen: quantitatively, it usually is in an inverse ratio to the degree of cellularity [5]. Mitotic figures, necrosis, and vascular infiltration are uncommon. A cellular vascular cuffing and the presence of perivascular inflammatory cells have been described by Allen [5]. The natural history comprises a proliferative phase with increased fibroblastic activity and cellular proliferation, an involutional phase, and a residual phase in which fibroblastic activity is reduced and maturation of the collagen is noted.
If this condition is treated by simple excision, there is a recurrence rate of 50–65 %, frequently resulting in a more aggressive form. Infiltration of neighboring structures is more frequently seen in recurrent lesions [5]. Wide, radical excision, encompassing 0.5 cm of surrounding, normal-appearing fascia, is, therefore, the treatment of choice.
On ultrasound images, plantar fibromatosis is seen as a well-defined, hypoechoic nodule or mass superficial to the medial slip of the plantar aponeurosis. Plantar fibromatosis exhibits a consistent and characteristic appearance on MR images [112]. Most lesions are heterogeneous, with an overall SI equal to or slightly higher than that of the muscle on both spin-echo T1- and (spin-echo and fast spin-echo) T2-weighted images (Fig. 13.20a, b). SI on T2-weighted images reflects the stage of the disease. The proliferative phase is characterized by higher cellularity, resulting in higher SI. The low SI on T2-weighted images of the involutional and residual phases is attributed to the hypocellularity and presence of abundant collagen. Lesions are slightly hyperintense to the muscle on short-tau inversion recovery (STIR) sequences (Fig. 13.20c). After intravenous injection of Gd contrast, enhancement is inversely related to the stage of fibromatosis. “Burned-out” lesions with abundant collagen fibers enhance to a lesser degree than lesions in the proliferative phase (Fig. 13.20e, f).
Differential diagnosis has to be made between plantar fibromatosis in the residual phase, callus, and scarring. Since plantar fibromatosis in the proliferative phase may be an extremely cellular and infiltrative lesion, it has to be differentiated from aggressive fibromatosis and fibrosarcoma. If the lesion is associated with palmar fibromatosis or Peyronie disease, or if both feet are involved, the diagnosis is almost certain [149].
13.3.3 Knuckle Pads
Knuckle pads consist of a flat or dome-shaped fibrous thickening on the dorsal aspect of the proximal interphalangeal or metacarpophalangeal joints. They are frequently associated with palmar and plantar fibromatosis. Microscopically, this condition resembles the latter fibromatosis.
On ultrasound, a platelike or ovoid thickening with a hypoechoic appearance is seen in the subcutaneous tissue superficial to the involved joints [19] (Fig. 13.21).


Fig. 13.21
Knuckle pads. Longitudinal ultrasound scans of finger PIP joints in two patients. Note a band-like diffuse thickening with a linear hypoechogenicity (a) and an ovoid, hypoechoic thickening (b) of the dorsal subcutaneous tissues overlying the interphalangeal joints. No distended joint capsule or increased joint fluid is seen, enabling the differentiation from synovitis (Reprinted with permission from Lopez-Ben et al. [94])
When confronted with periarticular soft tissue swelling at the small joints of the fingers, a clinician may confuse knuckle pads with synovitis as seen with rheumatological conditions: an ultrasound examination readily differentiates knuckle pads from synovitis [94]. MR imaging demonstrates focal thickening with intermediate signal intensity compared with muscle on T1- and T2-weighted sequences and with moderate enhancement [19].
13.3.4 Extra-abdominal Desmoid Tumors
Extra-abdominal desmoids are rare soft tissue tumors arising from the connective tissue of the muscle, overlying fascia, or aponeurosis. They have also been described as desmoid tumor, aggressive fibromatosis, or musculoaponeurotic fibromatosis. The term “desmoid” means band-like or tendon-like lesion [24].
The reported incidence of extra-abdominal desmoids is between three and four cases per million annually. The peak incidence is between ages 25 and 40 years. Although some authors report a predominance in women of childbearing age [31, 58], men and women are almost equally affected. Its cause is unknown, but surgical or accidental trauma, pregnancy, and estrogenic hormones are known associations. We have seen a recurrent desmoid tumor demonstrating an important increase in size (400 %) during pregnancy, while most desmoids regress after the menopause [136].
The localization of a large number of extra-abdominal desmoids at the lateral aspect of the shoulder and the buttocks supports the idea of a posttraumatic etiology. The notion of an inherited defect in connective tissue formation is supported by some authors, and a genetic basis for at least some desmoid tumors is suggested by Gardner’s syndrome. The term Gardner’s syndrome is used to describe extracolonic manifestations of familial adenomatous polyposis such as desmoid tumors, bony osteomas, dermoids and epidermoids, and/or nuchal fibromas together with abdominal wall desmoids and mesenteric desmoids [1, 148]. Some authors have hypothesized that an underlying mesenchymal defect may result in an imbalance in growth factors, resulting in multiple proliferative diseases, and may be responsible for the coexistence of desmoids, gastrointestinal polyps/colorectal cancer, and breast cancer [125]. Others have postulated a ciliary dysfunction as the underlying pathogenetic mechanism of the extracolonic manifestations in patients with familial adenomatous polyposis [54].
Extra-abdominal desmoids arise most commonly in the lower limb or limb girdle and in the shoulder region. In cases of lower limb localization, a preference for the region of the sciatic nerve is reported [148]. Our own series consists of 30 extra-abdominal desmoid tumors in 26 patients. The localizations were as follows: head and neck region (n = 1), upper limb (n = 4), trunk (n = 6), pelvis (n = 8), and lower limb (n = 11). Extra-abdominal desmoids never metastasize, but a multicentric, synchronous, and metachronous presentation has been reported [142, 156]. In this condition, second tumor localizations generally develop proximally to the primary lesion. In our series, 5 of 26 patients had multiple localizations.
Bone involvement is not uncommon and has been reported in up to 37 % of patients. In addition, primary intramedullary, juxtacortical, and periarticular desmoids are found, which are histologically indistinguishable from soft tissue desmoids. In the same way, bone desmoids can extend into the soft tissues just as soft tissue desmoids can involve the bone [27].
Microscopically, extra-abdominal desmoids consist of elongated spindle-shaped cells (fibroblasts) of uniform appearance, surrounded and separated from each other by varying amounts of collagen. Dense collagen is found at the periphery and fibroblasts at the center [81]. Conversely, the center of the lesions may contain dense collagen, whereas the periphery may be composed of fibroblasts [148]. In addition, myxoid change, focal hemorrhage, increased vascularity, and focal inflammation may be seen [81]. Immunohistochemistry staining was positive for actin, consistent with myofibroblastic differentiation in extra-abdominal desmoid tumors [156]. Despite their benign microscopic appearance, extra-abdominal desmoids have an aggressive behavior, which is expressed as large tumoral growth, infiltration of neighboring tissues, and a high incidence of postsurgical recurrence (between 25 and 68 %). Most recurrent tumors have a different behavior and morphology. In our series they show an increased frequency of extra-compartmental spread, grow considerably faster than primary tumors, and have a tendency to invade the bone more frequently.
In a retrospective study of 138 patients with extra-abdominal desmoids, 11 died as a consequence of locally uncontrolled tumor growth [124]. Kim [77] has retrospectively analyzed the clinical records and MRI findings in 40 patients (8 juveniles, 32 adults) with proven desmoid tumor. In his series, recurrences in the juvenile patients were more often multiple (50 % versus 12 %) and appeared significantly earlier than in the adult patients.
On ultrasound scans, extra-abdominal desmoids may be well-defined or poorly defined and may show variable echogenicity because of variable degrees of cellularity, matrix water content, and collagen. In many cases, marked shadowing from the surface of the lesion, a result of the large amount of dense collagen tissue in the tumor, totally obscures the mass [43].
Because of the variability in tumor composition, the lesions have variable attenuation and enhancement on CT scans [2]. Duda has reported that extra-abdominal desmoids are iso- or hypodense relative to the muscle and enhance to 100–110 Hounsfield units (HU) after injection of iodinated contrast material [28].
On MR images, the appearance of extra-abdominal desmoids varies [24, 117]. There are remarkable differences in shape in various patients as well as in different locations in a given patient with multicentric extra-abdominal desmoids. Likewise, for a given lesion, shape may vary on follow-up examinations. The infiltrative pattern is significantly more common in juvenile patients (63 %), whereas the nodular pattern is more frequent in the adult patients (81 %) [77, 106]. In our series, the shape of the lesions was fusiform-ovoid or dumbbell and irregular or stellate when located in the subcutis (Fig. 13.22). The mean maximum diameter was 72 mm. The “sunburst” extensions of subcutaneous desmoid tumors spread along the underlying fascia (fascial tail sign) and along the septa of the subcutaneous fat tissue, making complete surgical excision particularly difficult [106].


Fig. 13.22
(a, b) Desmoid of the upper arm (a) in a 23-year-old woman and (b) in a 44-year-old woman. (a) Axial spin-echo T1-weighted MR image. (b) Axial spin-echo T1-weighted MR image after gadolinium contrast injection. In the first patient, there is a stellar, infiltrating lesion within the subcutaneous fat of the deltoid region in close contact with the adjacent muscle fascia (a). In the second patient, there is a stellar, nodular infiltrating lesion within the subcutaneous fat of the deltoid region. The fat plane between the lesion and the adjacent muscle fascia is blurred (b). These two cases of superficial, stellar desmoid in the deltoid region with infiltration of neighboring fat and muscle fascia illustrate the characteristic shape and location of superficial aggressive fibromatosis
In the reported cases, extra-abdominal desmoids appear hypo- or isointense to the muscle on spin-echo T1-weighted images. SI on T2-weighted images is mostly intermediate, although very low and extremely high signal intensities have been noted occasionally. Low SI, patchy, linear, or curvilinear areas on T2-weighted images correspond to hypocellular tissue and dense collagen. On both spin-echo sequences, but predominantly on T2-weighted images, extra-abdominal desmoids are inhomogeneous, mostly with higher SI in the central part (cellular) than at the periphery (collagenous) [58, 81] (Figs. 13.23, 13.24, and 13.25).



Fig. 13.23
(a, b) Desmoid of the forearm in a 71-year-old man. (a) Axial spin-echo T1-weighted MR image. (b) Axial spin-echo T2-weighted MR image. On the T1-weighted image, there is a large mass at the volar aspect of the forearm adjacent to the interosseous membrane, with a rim of low signal intensity at the periphery of the lesion (a). On the T2-weighted image, the peripheral rim remains hypointense, while the central part is of higher signal intensity (b). This case illustrates a pattern of natural evolution where dense collagen is responsible for low signal intensity at the periphery of the lesion on all pulse sequences

Fig. 13.24




(a, b) Desmoid of the calf in a 23-year-old man. (a) Sagittal spin-echo T2-weighted MR image. (b) Sagittal spin-echo T1-weighted MR image with fat suppression after gadolinium contrast injection. On the T2-weighted image, there is a polylobular mass with central areas of low signal intensity within each nodule and presence of a low-signal-intensity capsule around the lesion (a). After contrast injection there is marked enhancement of the peripheral zones without enhancement of the central scar-like areas (b). This case of deeply seated juxtacortical desmoid (adjacent to the dorsal aspect of the tibial cortex) illustrates another pattern of natural evolution where collagen is found in the center and less collagenous parts at the periphery of the lesion. Furthermore, it illustrates a frequent finding that areas of higher signal intensity on T2-weighted images enhance after gadolinium administration, whereas areas of low signal intensity on T2-weighted images do not enhance and remain hypointense on all pulse sequences
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree