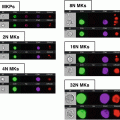
Fig. 1
FlowCam instrumentation. (a) Laboratory benchtop unit. (b) Portable unit
Table 1
Recommended phytoplankton cell size ranges (cell diameter), objective, and optimal analysis volume for different FlowCam flow cells
Cell size range (μm) | Objective | Flow cell | Optimal volume analyzed (mL) | Flow cell dimensions (Depth × width) (μm) |
|---|---|---|---|---|
Part number | ||||
FV = field of view | ||||
5–35 | 20× | FC50 | 2–5 | 50 × 1000 |
10–100 | 10× | FC80FV | 5–10 | 80 × 570 |
10× | FC80-7FV | 10 | 80 × 700 | |
10× | FC90-FV | 10 | 90 × 570 | |
10× | FC100 | 10 | 100 × 2000 | |
20–300 | 4× | FC300 | 25–150 | 300 × 3000 |
4× | FC300FV | 25–100 | 300 × 1500 | |
60–600 | 4× | FC600 | 100–200 | 600 × 6000 |
60–800 | 4× | FC800 | 100–300 | 800 × 8000 |
2.2 Sample Preparation
Environmental samples need to be unpreserved and analyzed within a couple of hours of sampling. Samples should be kept in the dark and cool (4–6 °C). A sterile filtered diluent needs to be prepared for cleaning the sample tubing and flow cell prior to analysis and for diluting samples that are too concentrated. Typically, 0.2 μm filtered fresh or salt water is used depending on the type of samples collected. If the only option is to preserve samples prior to processing, the samples should be preserved using a solution of 10 % buffered formalin solution (see Note 1 ), such that the final concentration is 1 or 2 %. Preserved samples need to be stored at 4 °C, in the dark and processed as soon as possible, a the algal pigment fluorescence within the cells decreases with prolonged sample storage. Auto Image Mode is recommended for best particle detection when working with preserved samples. For different environments the effects of preservation should be tested and compared to unpreserved samples. While formalin is the preferred method for phytoplankton preservation, the FlowCam can handle samples preserved by any method—Utermöhls, methanol, etc. (see Note 2 ).
3 Methods
3.1 Instrument Setup
The FlowCam is primarily used for nano- and micro-phytoplankton detection (cell size range 15–300 μm), and is ideally suited for analysis of both cultures and natural samples from diverse aquatic environments. It can also be used to both quantify phytoplankton and assist in the identification of phytoplankton to the genus level and in some cases to the species level.
1.
The instrument and laser should warm up for a minimum of 20 min, especially in older units.
2.
To detect standard fluorescent phytoplankton pigments (chlorophyll and phycoerythrin), the use of either a green or a blue laser instrument is required.
3.
The ViSp software is “set up” and threshold values for Trigger Mode detection (fluorescence detection) and particle cell size requirements need to be set within the “Context menu” of the ViSp software. Context settings are defined by the operator, and will be based on the phytoplankton cell size, sample type, location, and depth (see Note 3 ).
4.
5.
The FlowCam needs to be focused prior to initiating a run, this can be done manually for older instruments, or automatically using the ViSp software (purchased as an option with new instruments). Fluorescent or non-fluorescent latex beads can be used to focus the instrument prior to sample analysis.
6.
The FlowCam pump (peristaltic or syringe) needs to be primed with the appropriate sample diluent (fresh or salt water).
3.2 Modes of Operation
The FlowCam VS-4 is equipped with two primary modes of operation, Trigger Mode and Auto Image Mode. Trigger Mode uses a laser as the primary tool for detecting particles via fluorescence or scatter detection. For determining the presence of algae in a natural sample the algal pigments (chlorophyll and/or phycoerythrin) are detected by auto-fluorescence using laser light allowing for image capture. In the case of Trigger Mode the density of the phytoplankton needs to be ~1–1.5 cells per camera field of view. As the cells are imaged and archived by the ViSp software the particles per image are calculated and updated.
If dense or concentrated phytoplankton samples need to be analyzed, such as phytoplankton cultures, Auto Image Mode is the preferred mode of detection. Auto Image Mode does not use the laser for cell or particle detection. In this mode the camera is set at a specified frame rate ranging from 0 to 20 frames per second. When the camera shutters, images of all particles or cells within the camera’s field of view are captured and saved throughout the sample run. In Auto Image Mode the number of camera images captured is determined and compared to the volume of sample analyzed and the ViSp Software determines the concentration of the cells in the sample.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree