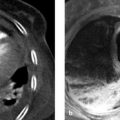
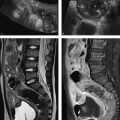
Anatomy
6.1.1 Biliary Fluid
Biliary fluid is composed of 85% water, 10% bile salts, 3% glycoproteins, 1% fat, and 0.7% inorganic salts. A total volume of approximately 1 liter of this “liver bile” is produced daily. 1 When the papilla is closed, the initially low-viscosity liver bile is stored in the gallbladder where water is absorbed from the fluid, raising its viscosity and concentrating it to approximately 10% of its initial volume (100 to 150 mL).
When lipids enter the small intestine, they stimulate production of the hormone cholecystokinin in the intestinal mucosa. Cholecystokinin stimulates the smooth muscle of the gallbladder wall, causing it to contract and release stored bile into the duodenum. Increased vagus nerve activity has the same effect.
The bile enables fat digestion by emulsifying ingested lipids, making them accessible to attack by lipases. Moreover, water-insoluble substances and toxins are excreted in the bile. Another important function is neutralization of the acidic gastric juice by the alkaline bile.
Bile derives its characteristic color from the bile pigments bilirubin and biliverdin, which are formed from the breakdown of porphyrins and hemoglobin in the hepatocytes. Bile acids, bilirubin, and cholesterol participate in the enterohepatic circulation; 90% of these substances are absorbed in the terminal ileum and transported back to the liver through the portal vein. This recirculation process maintains a total bile salt pool of 2 to 4 g in the body, which is sufficient to meet the fat absorption requirement of 20 to 30 g/day.
6.1.2 Biliary Capillaries and Bile Ducts
The functional units of the liver, the lobules, are approximately 2 mm high and 1 to 1.5 mm in diameter and are arranged in a honeycomb pattern. Branches of the portal vein and hepatic artery are found at the corners of each lobule. Both vessels supply a three-dimensional space composed of specialized capillaries, called sinusoids, which are permeated by cords of hepatocytes. The sinusoids drain into the central vein located at the center of each lobule, so that the blood flow is directed from the periphery of the lobule toward its center. 2
The system of bile ducts begins between the walls of adjacent lobules in the form of bile canaliculi 0.1 to 1 mm in diameter. The canaliculi between two hepatocytes are arranged in such a way that part of the hepatocyte is interposed between the biliary network and sinusoids. This creates a physical separation between the biliary network and vascular system at the cellular level.
The flow of biliary fluid is directed oppositely to the blood flow, i.e., from the center of the lobule toward its periphery (▶ Fig. 6.1a). Special short connecting channels at the edge of each lobule, called the canals of Hering (30 μm in diameter), interconnect the bile capillary network with the interlobular bile ducts.

Fig. 6.1 Flow of bile and blood in the liver. Schematic representation. (a) Bile flows from the center to the periphery of the hepatic lobules, opposite to the direction of blood flow. (b) The small ducts merge at the portal triad, following the distribution of the portal vein, and unite to form increasingly larger ducts. (c) The segmental ducts unite to form the right and left hepatic ducts and finally the common hepatic duct. Roman numerals indicate the Couinaud segments of the liver. (Reproduced from Schünke M, Schulte E, Schumacher U. Prometheus. LernAtlas der Anatomie: Innere Organe. Illustrated by M. Voll/K. Wesker. 2nd ed. Stuttgart: Thieme; 2009.)
The interlobular bile ducts accompany the intrahepatic branches of the portal vein and hepatic vein. Together these vessels form the “portal triad” (Glisson’s triad), which is embedded in periportal connective tissue (▶ Fig. 6.1b). Following the branching pattern of the portal vein, they merge to form increasingly larger ducts that finally become the ducts of the hepatic segments. The segmental ducts of the hepatic right lobe unite to form the right main trunk, while those of the left lobe join to form the left main trunk: the right and left hepatic ducts (▶ Fig. 6.1c). The common hepatic duct is formed by the junction of the right and left ducts, which may unite within the liver parenchyma or just after emerging from the liver. The common hepatic duct is 3 to 4 cm long and 6 mm in diameter.
The extrahepatic duct formed by the union of the common hepatic duct and cystic duct is called the common bile duct (ductus choledochus). Measuring approximately 4 to 8 cm long and 6 mm in diameter, the common bile duct is divided into a supraduodenal segment and a retroduodenal (or pancreatic) segment (▶ Fig. 6.2a).

Fig. 6.2 Common bile duct. Schematic representation. (a) Course of the extrahepatic bile ducts. The arrows indicate the direction of bile flow from the gallbladder. (b) Duodenal papilla. (c) Variations of the duodenal papilla. (Reproduced from Schünke M, Schulte E, Schumacher U. Prometheus. LernAtlas der Anatomie: Innere Organe. Illustrated by M. Voll/K. Wesker. 2nd ed. Stuttgart: Thieme; 2009.)
The common bile duct terminates at the papilla of the duodenum. The papilla is a muscular valve that controls the passage of bile and pancreatic secretions into the small intestine. Its closure mechanism consists of three sphincters, one each for the bile duct and pancreatic duct plus a common sphincter in the duodenal wall called the sphincter of Oddi (▶ Fig. 6.2b). The oblique course of the ducts through the intestinal wall helps to prevent the reflux of small bowel secretions into the biliary tree. In 70% of cases the terminal segments of the common bile duct and main pancreatic duct unite at a common junction, the ampulla of Vater. In 10% of cases the common bile duct and main pancreatic duct enter the duodenum at separate sites. In the remaining 20% the ducts unite to form a common long terminal segment (▶ Fig. 6.2c). 3
6.1.3 Gallbladder
The gallbladder is connected to the common hepatic duct by the cystic duct. It occupies a small fossa on the undersurface of the liver at the junction of the right and left lobes and is fused to the liver at that location. The gallbladder’s surface facing the abdominal cavity is covered by peritoneum. The gallbladder extends from the porta hepatis to the anterior border of the liver. It consists of a blind pouch (the fundus), a body, and a neck (see ▶ Fig. 6.2a). The fundus extends down past the inferior border of the liver in many cases. The body of the gallbladder is in contact with the duodenum and right colic flexure. The gallbladder neck, from which the cystic duct arises, is adjacent to the porta hepatis.
Note
A spiral arrangement of mucosal folds in the initial part of the cystic duct prevents the passive drainage of bile from the gallbladder but does allow bile to enter from the common hepatic duct when the papilla is closed. 1
The gallbladder has a total capacity of 40 to 50 mL. Its volume is variable owing to the specialized structure of the gallbladder wall.
6.1.4 Blood Vessels, Lymphatics, and Nerves
The gallbladder receives its blood supply (▶ Fig. 6.3) from the cystic artery, which generally arises from the right hepatic artery (see ▶ Fig. 6.3a). Possible variations are shown in ▶ Fig. 6.4. A normal variant in which the right hepatic artery arises from the superior mesenteric artery is clearly demonstrable by CT. Normal variants in the origin of the cystic artery itself can be demonstrated only by conventional angiography. Venous blood is drained by the cystic vein to the portal vein or directly to the intrahepatic portal vein (see ▶ Fig. 6.3c). The portal vein occupies a far posterior position in the hepatoduodenal ligament, just behind the hepatic artery. The bile ducts are anterior (see ▶ Fig. 6.4a). The intrahepatic bile ducts are supplied by accompanying branches of the hepatic artery and portal vein.

Fig. 6.3 Blood vessels of the gallbladder. (a) Selective visualization of the right hepatic artery and cystic artery (arrow). (b) Opacification of the hepatic right lobe and gallbladder wall in the parenchymal phase. (c) Indirect portography via the celiac trunk defines the veins of the gallbladder (arrow), which drain into the right main branch of the portal vein.

Fig. 6.4 Variants in the arterial blood supply of the gallbladder. (a) Normal origin of the cystic artery from the right hepatic artery. The vessel may be single (left) or duplicated (right). (b) Normal variant in which the cystic artery arises from the gastroduodenal artery (left) or from an aberrant right hepatic artery (right). (c) Dual blood supply from the right hepatic artery and gastroduodenal artery.
The lymphatic vessels of the gallbladder communicate with those of the liver. The regional lymph nodes are located at the porta hepatis in the hepatoduodenal ligament.
The gallbladder and bile ducts derive their autonomic nerve supply from sympathetic and parasympathetic fibers of the hepatic plexus.
6.2 Physiology and Pathophysiology
6.2.1 Cholestasis (Obstructive Jaundice)
Cholestasis results from a disturbance of bile secretion leading to the retention of substances such as bilirubin and bile acids. The clinical hallmark of cholestasis is jaundice accompanied by generalized pruritus; the itchiness is caused by the deposition of bile acids in the skin. Pruritus may be absent in some forms of intrahepatic cholestasis. Obstructive jaundice is characterized by beer-brown urine and pale (acholic) stools. Laboratory tests show hyperbilirubinemia and elevated alkaline phosphatase activity.
Note
Except for rare hereditary forms (Alagille’s syndrome and various forms of progressive familial intrahepatic cholestasis), cholestatic jaundice is always acquired.
Cholestatic jaundice can have two main causes 4 (▶ Table 6.1):
Nonobstructive cholestasis: caused by a primary disturbance of bile secretion in the hepatocytes themselves.
Obstructive cholestasis: caused by a mechanical outflow obstruction in the common hepatic duct or common bile duct.
Table 6.1 Acquired cholestatic jaundice
Pathophysiology
Examples
Intrahepatic cholestasis
Caused by impaired bile secretion from hepatocytes without dilatation of the bile ducts
All forms of cirrhosis
All cholestatic hepatitis
Drug-induced jaundice
Sepsis
Caused by diffuse impairment of drainage in the liver with dilatation of the intrahepatic bile ducts only
Primary biliary cirrhosis
Caused by proximal intrahepatic obstructions with dilatation of the intrahepatic bile ducts only
Metastases and liver tumors
Cholangiocellular carcinoma (Klatskin’s tumor)
Cholangitis, especially oriental cholangiohepatitis
Inflammatory or infiltrative diseases of the periportal fields
Hepatolithiasis secondary to hepatic cirrhosis
Extrahepatic cholestasis
Caused by distal extrahepatic obstructions with dilatation of the extrahepatic and intrahepatic bile ducts
Choledocholithiasis
Biliary sludge
Mirizzi’s syndrome
Biliary stricture
Common bile duct carcinoma
Gallbladder carcinoma
Common bile duct metastasis
Choledochal cyst
Papillary carcinoma
Pancreatic carcinoma
Pancreatitis (chronic, acute)
Duodenal diverticulum
Biliary atresia
Extrinsic compression by masses in the hepatoduodenal ligament
The unilateral obstruction of a hepatic duct usually does not cause jaundice, as the unaffected half of the liver can still maintain an almost normal level of bilirubin excretion.
Intrahepatic nonobstructive cholestasis is most commonly caused by impaired secretion from hepatocytes and occurs in the following entities, among others:
All forms of cirrhosis.
Cholestatic viral hepatitis.
Drug-induced cholestatic jaundice.
Cholestatic steatohepatitis.
The bile ducts are not dilated in any of these conditions. On the other hand, jaundice caused by a mechanical outflow obstruction is almost always associated with increasing dilatation of the bile ducts (▶ Fig. 6.5).

Fig. 6.5 Dilatation of the bile ducts. (a) Ultrasound shows initial intrahepatic biliary obstruction with unilateral thickening of periportal fields (arrows). (b) Symmetrical intrahepatic biliary obstruction on axial CT. (c) Obstructive jaundice due to pancreatic head carcinoma. Contrast medium was instilled through an external biliary drain.
6.3 Imaging
6.3.1 Conventional Radiography
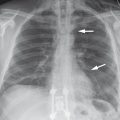
The abdominal plain film is not suitable for evaluating diseases of the biliary system. However, calcified gallstones, calcifications of the gallbladder wall (porcelain gallbladder), and air in the bile ducts (▶ Fig. 6.6), gallbladder, or gallbladder wall may be visible on radiographs obtained for a different indication.

Fig. 6.6 Pneumobilia. Rotated view in the left lateral decubitus position.
6.3.2 Ultrasonography
Initial Ultrasound Examination
Ultrasound and MRI are the principal modalities for biliary tract imaging, providing an almost 100% detection rate for gallstones and acute cholecystitis.
The diameter of the common bile duct does not exceed 6 to 7 mm in a healthy individual. The normal intrahepatic bile ducts are always smaller than the accompanying portal vein branches. The right and left hepatic ducts can be defined with ultrasound and are approximately 2 mm in diameter. Dilatation of the intrahepatic bile ducts is characterized initially by a unilateral thickening of the periportal connective tissue.
Note
With increasing dilatation, the intrahepatic bile ducts can be identified as a second linear duct system accompanying the portal vein branches in the periportal connective tissue. This phenomenon is called the “double-barrel shotgun sign” and is also known as the “parallel channel sign” (see ▶ Fig. 6.5a).
Ultrasound Evaluation of Gallbladder Function
Ultrasound can be used to evaluate gallbladder emptying (▶ Fig. 6.7) after a cholecystokinin injection or the ingestion of a provocative meal (with chocolate). Gallbladder volume is measured sonographically in the fasting state and again after stimulation. Volume determination is based on the assumption of a rotational ellipsoid using a simplified geometric formula:

Gallbladder volume should decrease by at least 50% in response to the provocative meal.

Fig. 6.7 Ultrasound evaluation of gallbladder function. (a) Fasting gallbladder volume is 35 mL in a patient with cholecystolithiasis. (b) The gallbladder shows normal volume reduction (12 mL) after a provocative meal. (c) Fasting gallbladder volume is 63 mL in a patient with sludge in the infundibulum (arrow). (d) Emptying does not occur (56 mL) in response to a provocative meal.
6.3.3 Computed Tomography
Computed tomography (CT) is less sensitive than ultrasound in the detection of gallstones. The density of gallstones may vary with their composition and ranges from fat attenuation to dense calcification. Approximately 20% of gallstones are isodense to biliary fluid and therefore are not visualized on CT scans. The detection rate for gallbladder sludge is even lower.
The strength of CT lies in its ability to define the tissues around the gallbladder. CT is also better than ultrasound for detecting masses of the bile ducts, gallbladder, and pancreas and for identifying secondary tumor signs. CT is the imaging modality of choice for the evaluation and staging of pancreaticobiliary masses.
6.3.4 Magnetic Resonance Imaging and Magnetic Resonance Cholangiopancreatography
Heavily T2-weighted sequences can be used to visualize the fluid-filled bile ducts (and the pancreatic duct) with magnetic resonance imaging (MRI). In contrast to direct visualization of the ducts with contrast material, conventional MRI can simultaneously demonstrate the parenchyma and vasculature along with the bile ducts. MRI is somewhat better than CT for imaging tumors of the biliary tract.
The spatial resolution of magnetic resonance cholangiopancreatography (MRCP) is less than that of direct visualization with contrast material in endoscopic retrograde cholangiopancreaticography (ERCP). One limitation of MRI is its susceptibility to artifacts from indwelling biliary drains or stents.
Note
Imaging of the biliary tree with MRI is significantly improved by administering secretin, which stimulates distension of the ducts.
The level of detail can be improved by the administration of liver-specific contrast agents that are excreted in the bile.
6.3.5 Percutaneous Transhepatic Cholangiodrainage
Percutaneous transhepatic cholangiodrainage (PTCD) is important in the treatment of biliary obstruction. It is used in cases where the bile ducts are not accessible endoscopically (e.g., after a Whipple operation) or if an obstruction cannot be crossed from the duodenal side.
In these cases the obstruction is reached and crossed percutaneously via the intrahepatic bile ducts. This provides access for the implantation of a suitable biliary stent, allowing the temporary drain to be removed (“internalized” drainage by stent placement).
6.4 Normal Variants, Developmental Anomalies, and Congenital Disorders
Various disorders, known collectively as fibropolycystic liver disease, may develop depending on the location of the developmental abnormality in the biliary tract (▶ Fig. 6.8):
Congenital hepatic fibrosis: abnormal development of bile ducts in the hepatic lobules.
Biliary hamartomas of interlobular ducts: microhamartomas, von Meyenburg’s complex.
Autosomal dominant polycystic liver disease: small ducts that are separate from the actual duct system (▶ Fig. 6.9).
Caroli’s syndrome: cystic dilatation of large (segmental) ducts with hepatic fibrosis.
The Todani system is used to classify malformations involving cystic dilatation of the common bile duct and/or the intrahepatic ducts (▶ Fig. 6.10) 6:
Todani type I: dilatation of the common bile duct (which may extend into the common hepatic duct and main ducts).
Todani type II: diverticulum of the common bile duct.
Todani type III: choledochocele (dilatation of the intramural small-bowel segment with herniation in the papillary region).
Todani type IV: dilatation of intra- and extrahepatic bile ducts.
Todani type V: cystic dilatation of intrahepatic bile ducts (synonym: Caroli disease or Caroli syndrome when hepatic fibrosis is also present).

Fig. 6.8 Fibropolycystic liver disease. The specific disease depends on the anatomical location of the affected bile ducts. In autosomal dominant polycystic liver disease, the cysts form in small ducts that are separate from the actual duct system. 5

Fig. 6.9 Autosomal dominant polycystic liver disease. Ductal malformation characterized by small, isolated ducts in two different patients. Patient 1 has adult polycystic liver change with branching cysts, some with a ductal appearance ([a] and [b], arrows). Patient 2 has a milder case with an associated anomaly (solitary biliary hamartoma; [d], arrow). (a) Patient 1. (b) Patient 1 (adjacent slice). (c) Patient 2. (d) Patient 2 (adjacent slice).

Fig. 6.10 Todani classification of choledochal cysts. Type I, cystic dilatation of the common bile duct, with possible extension into the cystic duct and right or left hepatic duct. Type II, diverticulum of the common duct. Type III, choledochocele. Type IV, dilatation of intra- and extrahepatic bile ducts. Type V, cystic dilatation of intrahepatic bile ducts.
6.4.1 Anatomical Variants
Variants of the Gallbladder
Brief definition Variants of gallbladder morphology are congenital and may involve the presence of internal septa (▶ Fig. 6.11a) or angulations. The most familiar congenital morphological variant of the gallbladder is the Phrygian cap, in which the fundus is elongated and folded over (▶ Fig. 6.11b). Morphological variants are incidental imaging findings that do not have pathologic significance.

Fig. 6.11 Morphological variants of the gallbladder. (a) Septum within the gallbladder. Longitudinal ultrasound scan. (b) Phrygian cap. Axial fat-suppressed T2W MRI, maximum intensity projection (slice thickness 4 cm).
Note
Circumscribed constrictions or diverticula of the gallbladder are predisposing factors for gallstone formation.
The cystic duct is also variable in its course and termination. For example, the cystic duct may run parallel to the common hepatic duct for some distance before joining it at the level of the pancreas just above the papilla (▶ Fig. 6.12). In other cases the cystic duct may open immediately into the right hepatic duct. Rarely, accessory bile ducts may arise from the liver and enter the gallbladder.

Fig. 6.12 Variants of the cystic duct. (a) Schematic diagrams showing possible variations in the course and termination of the cystic duct. (b) Endoscopic view. Low termination of the cystic duct.
Variants of the Bile Ducts and Biliary Vessels
Note
Anatomical variants of the biliary system have no pathologic significance. Their danger lies in their possible misinterpretation at imaging. Unless normal duct variants are identified and described, there is a potential for duct injury or ligation of the wrong duct during laparoscopic cholecystectomy. Duct anomalies may also be significant if liver transplantation is planned.
Brief definition The posterior duct of the hepatic right lobe that drains segments VI and VII shows the most variable termination (15% incidence of variants in the general population). It may open into the left main duct (“crossed anomaly,” ▶ Fig. 6.13a), into the confluence of the right and left main ducts (“triple confluence,” ▶ Fig. 6.13b), or less commonly into the cystic duct, the common bile duct, the gallbladder, or even the pancreatic duct (▶ Fig. 6.14). A frequent anomaly of the cystic duct is a “long cystic duct” that joins the common hepatic duct at a low level close to the papilla. In other cases the cystic duct may open into the right or left hepatic duct (▶ Fig. 6.12a). 7 Variations in the arterial supply to the gallbladder may have surgical implications. For example, the cystic artery may arise from the left hepatic artery or from the proper hepatic artery. The biliary artery may have multiple trunks. The right hepatic artery may be misidentified as the cystic artery (see ▶ Fig. 6.4). 3

Fig. 6.13 Variants in the termination of the right posterior duct. The solid arrows indicate the right posterior duct; the dashed arrows indicate the anterior duct. (a) The right posterior duct opens into the left hepatic duct: crossed anomaly. (b) The duct opens into the confluence of the left and right anterior ducts: triple confluence.

Fig. 6.14 Aberrant bile duct opening into the pancreatic duct in a woman who underwent cholecystectomy for recurrent attacks of biliary pancreatitis. (a) Aberrant duct (solid arrow) and cystic duct stump (dashed arrow) behind the dilated common hepatic duct. (b) Aberrant duct (arrow), dilated major pancreatic duct (dashed arrow). (c) MRCP shows the posterior right duct inserting into the pancreatic duct (arrow). The rest of the biliary tree is dilated. (d) Pancreatogram shows opacification of the right posterior duct and cystic duct stump (arrow). Note the associated anatomical variant of the pancreas. C, venous confluence; H, common hepatic duct; P, portal vein.
Imaging signs
Normal variants of intra- and extrahepatic bile ducts: Normal variants of these ducts can be recognized during magnetic resonance cholangiopancreatography (volume-rendered MRCP images, maximum intensity projection, respiratory-triggered 3D TSE sequence) or during endoscopic retrograde cholangiopancreaticography (ERCP). CT cholangiography provides the highest spatial resolution and can be used for special investigations (e.g., before liver transplantation) in cases where MRCP findings are equivocal or MRI is contraindicated.
Normal variants of extrahepatic bile ducts: These ducts can also be identified in a detailed analysis of axial MR images (heavily T2-weighted sequences, T2W or T1W TrueFISP sequences) or thin-slice CT scans.
Normal variants of blood vessels: Besides conventional angiography, thin-slice arterial CT angiography (CTA) images are best for demonstrating normal variants of vascular anatomy.
Key points It is common to find normal variants in the bile duct and vascular anatomy. Generally they have no pathologic significance but may become important during laparoscopic gallbladder procedures or transplantation surgery.
6.4.2 Biliary Atresia
Brief definition The prevalence of biliary atresia is approximately 1 in 10,000 to 13,000 live births. Intrahepatic ductal atresia (either syndromic or nonsyndromic) is much less common than atresia of extrahepatic bile ducts. Extrahepatic atresia may be confined to the common bile duct or may additionally affect the common hepatic duct. Atresia of both ducts is very rare. The gallbladder is present in one-fourth of cases. The cause of biliary atresia is not fully understood but presumably relates to a prenatal viral infection of the biliary tract leading to destructive inflammatory obliteration of the bile ducts. Thus, the disease is not a congenital absence of duct systems but a gradually progressive process that develops in postnatal life. 8 Approximately one-fourth of patients have associated congenital disorders (polysplenia syndrome, cardiac anomalies, portal vein anomalies).
Imaging signs The initial imaging study in newborns is ultrasound. The echogenicity of the liver is initially normal. The liver may already be enlarged at birth but will definitely enlarge during postnatal development. The common bile duct is invariably absent. In rare cases imaging may demonstrate the right and left hepatic ducts, the common hepatic duct, or dilated intrahepatic segments of the largely obliterated duct system (“bile lakes”). The common duct is replaced in the porta hepatis by an echogenic band. A gallbladder is demonstrable in 25% of cases. When present, it has an unusual shape and shows irregular wall thickening (▶ Fig. 6.15). Functional ultrasonography (initial scan after a 4-hour fast; rescan after nursing) shows there to be no gallbladder contraction. Untreated patients will develop hepatic cirrhosis and portal hypertension. Both of these conditions are also diagnosed sonographically. Hepatobiliary scintigraphy (technetium Tc 99m HIDA sequential scintigraphy) requires 5 days’ preparation with phenobarbital (oral) to stimulate bile secretion from hepatocytes. Entry of the tracer into the small intestine excludes biliary atresia. Absence of detectable tracer in the small intestine by 24 hours confirms obstructive jaundice, which may be caused by biliary atresia (see ▶ Fig. 6.15b) or extrinsic compression of the common duct. The presence of inspissated bile should also be considered, especially in premature infants. CT is contraindicated in this age group due to radiation exposure concerns and the need for sedation.

Fig. 6.15 Biliary atresia in a 1-month-old boy. (a) Bandlike widening of the periportal field at the site of the common hepatic duct (arrows). The gallbladder (dashed arrow) is small and shows irregular wall thickening. (Image [a] with kind permission of Professor Berthold, Hannover Medical School, Germany.) (b) Hepatobiliary scintigraphy. Image at 6 hours shows hepatic tracer uptake with no excretion into the bowel. Tracer in the bladder (arrow) results from ectopic excretion. (Image [b] with kind permission of Dr. Steiner, Nuclear Medicine, University Hospital Giessen, Germany.) (c) Only the pancreatic duct is visualized on ERCP. (Image [c] with kind permission of Dr. Collet, University Hospital Giessen, Germany.)
Caution
Because the absence of extrahepatic bile ducts is the hallmark of biliary atresia, MRCP is of very limited value. Even normal bile ducts may be difficult to detect in infancy.
Both CT and MRCP can aid in the differentiation of extrinsic common bile duct compression, however. Nonvisualization of the common bile duct with a visible pancreatic duct on ERCP provides high diagnostic accuracy (see ▶ Fig. 6.15c). If the common bile duct can be visualized, there is no atresia.
Clinical features Affected children are born with normal body weight at term or occasionally before term. Approximately one-third of newborns are already jaundiced at birth, and jaundice will gradually develop in the rest. Because the jaundice has a mechanical causation, acholic stools and dark-brown urine are present. A progressive rise of conjugated bilirubin is found. The liver enlarges over time, with rapid development of hepatic cirrhosis. It takes just 2 months for irreversible liver damage to occur. Untreated children will die from biliary hepatic cirrhosis within 12 to 15 months. The only treatment option is a biliary–enteric anastomosis. In 10% of children the atresia affects only the common bile duct, allowing the hepatic duct to be used for a biliary–enteric reconstruction. The remaining 90% of atresias can be treated with a Kasai’s hepatic portoenterostomy. 9 This procedure is 90% successful when performed during the first 2 months of life. When done after 3 months, its success rate falls to less than 50%. Even patients who have the Kasai’s operation will eventually require liver transplantation.
Differential diagnosis
Neonatal hepatitis: This is the most frequent cause of neonatal jaundice besides physiologic jaundice (possible causes are hepatitis A and B, cytomegalovirus infection, rubella, or toxoplasmosis).
Bile plug syndrome: This may occur in cystic fibrosis, sepsis, or total parenteral nutrition.
Atresia of intrahepatic bile ducts: Alagille’s syndrome, common duct malformation, and obstructive jaundice due to extrinsic compression of the common duct (hydronephrosis, duodenal atresia, etc.).
Key points Biliary atresia is a rare but important differential diagnosis in newborns with persistent obstructive jaundice and in infants with progressive obstructive jaundice. It is not a primary congenital anomaly but results from inflammatory destruction of the bile ducts. The key to diagnosis is nonvisualization of the common bile duct on ultrasound. Visualization of the gallbladder does not exclude biliary atresia. The diagnosis is confirmed by hepatobiliary scintigraphy and endoscopy. The only effective treatment option is hepatic portoenterostomy (Kasai’s operation) before 2 months of age. Ten percent of cases can be treated with a simple biliary–enteric anastomosis.
6.4.3 Choledochal Cysts
Brief definition Five types of choledochal cyst are distinguished by their site of occurrence in the Todani classification (see ▶ Fig. 6.10). Cyst size varies from 1 cm to more than 15 cm. The prevalence of choledochal cysts is approximately 1:100,000 live births in the United states and 1:1,000 live births in Japan. Approximately one-third of all documented cases originate in Japan. Girls are affected more frequently than boys. Three causal factors are considered:
Failure of recanalization. 10
Pancreaticobiliary reflux due to a long, common terminal segment. 11
Distal obstruction. 10
Approximately one-half of infants become symptomatic before 1 year of age, but complaints may not appear until much later in life. 12
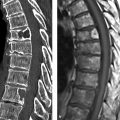
Imaging signs Ultrasound is ideal for revealing dilatation of the extra- and intrahepatic bile ducts. If the diameter of the common bile duct is greater than 10 mm in childhood, the most likely cause is a choledochal anomaly (▶ Fig. 6.16). MRCP is the technique of choice for noninvasive ductal imaging. Hepatobiliary scintigraphy can be used for functional evaluation.

Fig. 6.16 Todani type I choledochal cyst in a 10-year-old boy with upper abdominal pain. (a) The common bile duct is dilated to 4 cm. (b) Sludge in the common bile duct. (c) Maximum transverse diameter of the common bile duct. (d) Major pancreatic duct is dilated and opens into a long, common terminal segment (arrow). (e) Dilatation of the common bile duct extends into the cystic duct. (f) Fat-suppressed T1W MRI after administration of contrast medium shows a filiform choledochal stenosis (dashed arrow) just proximal to its junction with the pancreatic duct (solid arrow).
Clinical features Infants younger than 12 months show intermittent obstructive jaundice combined with hepatomegaly and vomiting. Sixty percent of older children and adults manifest the classic triad of upper abdominal pain, jaundice, and a right-upper-quadrant mass. The remaining 40% have nonspecific upper abdominal complaints that may be accompanied by fever, intermittent jaundice, nausea, and vomiting. Stones are found in 30% of adult patients. 13 Pancreatitis may also occur. Biliary cirrhosis may be present as an initial diagnosis. Patients with a choledochal cyst are at increased risk for the development of bile duct cancer (cholangiocarcinoma).
Treatment decisions are based on the Todani classification:
Type I: complete surgical resection with biliary–enteric reconstruction (typically in the form of a Roux-en-Y choledochojejunostomy).
Type II: resection of the diverticulum, preserving the biliary tree.
Type III: endoscopic papillotomy, or open transduodenal removal for larger cysts.
Type IV: resection tailored to the location and extent of the dilatations.
Type V: options ranging from segmental resection to hemihepatectomy; the diffuse type requires liver transplantation.
Differential diagnosis
Chronic cholangitis with stricture and secondary cystic ductal dilatation.
Obstructive cholelithiasis.
Pancreatic pseudocysts.
Echinococcal cysts.
Key points Choledochal anomalies in children are easily detected sonographically and are diagnosed when the diameter of the common bile duct is 10 mm or more. Choledochal cysts may be as large as 15 cm. In one-half of children the anomaly becomes symptomatic during the first year of life, but many cases are not diagnosed until adulthood. Symptoms are obstructive jaundice, an abdominal mass, and recurrent episodes of cholangitis. Possible complications are lithiasis or even biliary pancreatitis. Patients with choledochal cysts are at increased risk for developing cholangiocarcinoma.
6.4.4 Caroli’s Disease and Caroli’s Syndrome
Brief definition Since intrahepatic segmental bile duct cysts were first described by Caroli in 1958, two main types of disease have been distinguished 1:
Caroli’s disease: a simple but very rare type without associated hepatic fibrosis.
Caroli’s syndrome: a congenital type of fibrosis.
The simple form results from anomalous development of the large, central bile ducts. Its cause is not known. Caroli’s disease, unlike Caroli’s syndrome, occurs sporadically. It consists of nonobstructive, cavernous dilatations of intrahepatic bile ducts (diffuse, lobar, or segmental). If all portions of the intrahepatic biliary system are affected and cystic dilatation of the ducts is accompanied by congenital fibrosis, the condition is Caroli’s syndrome. Caroli’s syndrome has an autosomal recessive mode of inheritance and is associated in 60% of cases with autosomal recessive polycystic kidney disease. 14
Imaging signs The principal imaging sign is intrahepatic biliary obstruction with the cystic dilatation of bile ducts, which is clearly demonstrable by ultrasound. Intraductal sludge or gallstones may be found within the intrahepatic ducts. The portal vein branches may pass through areas of cystic duct dilatation 15; this finding can be confirmed by duplex ultrasound scanning. Caroli’s syndrome may also be associated with signs of hepatic cirrhosis and portal hypertension.
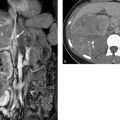
Contrast-enhanced CT defines the extent and distribution of the ductal dilatation. The presence of opacified portal vein branches within the dilated intrahepatic ducts (“central dot sign”) is considered pathognomonic. 16 CT is less effective than ultrasound or MRI in the detection of intrahepatic gallstones. Cyst contents of high attenuation should suggest hepatic abscess. CT can also diagnose hepatic cirrhosis and its sequelae, splenomegaly with portomesenteric varices. MRI (using T2W sequences and contrast-enhanced T1W sequences) can detect cystic dilatation of the intrahepatic ducts in addition to the presence of portal vein branches within the dilatations (▶ Fig. 6.17). MRCP has the ability to detect intrahepatic gallstones and biliary strictures due to scarring. 17

Fig. 6.17 Caroli’s syndrome. (a) T2W image shows multiple hyperintense, oval, saccular dilatations of small intrahepatic bile ducts. (b) The intrahepatic ducts are only slightly dilated. (c) Axial maximum intensity projection. (d) Coronal maximum intensity projection. (Images with kind permission of Prof. Berthold, Hannover Medical School, Germany)
Clinical features The simple type, Caroli’s disease, is much rarer than the fibrosing type, Caroli’s syndrome. Both types may become clinically apparent at any age, but most patients become symptomatic in the second decade of life. The most common presenting symptom is right upper quadrant pain. Simple Caroli’s disease presents with recurrent episodes of cholangitis with fever and jaundice. Patients develop intrahepatic duct stones and hepatic abscesses. The clinical manifestations of Caroli’s syndrome are determined by hepatic cirrhosis and portal hypertension. Cholangitis is less common and usually occurs only after portal hypertension has developed. 1 The risk of cholangiocarcinoma is increased by a factor of 100 (ca. 7% of patients develop bile duct cancer). Intrahepatic cholelithiasis can be treated conservatively with ursodeoxycholic acid. 18 The first-line treatment option for cholangitis is antibiotic therapy. Localized forms should be treated surgically (by lobectomy or segmentectomy). Drainage may be necessary for biliary decompression. Liver transplantation is indicated for patients with refractory cholangitis or biliary cirrhosis.
Differential diagnosis
Polycystic kidney disease.
Primary sclerosing cholangitis.
Recurrent suppurative cholangitis and hepatic abscess.
Biliary hamartomas.
Key points Simple, nonobstructive dilatation of the large intrahepatic ducts (Caroli’s disease) is distinguished from a congenital fibrosing type that additionally involves the small ducts (Caroli’s syndrome). There is also a pure congenital fibrosing type in which only the small ducts are affected. Presentation in early adulthood is common. The cardinal symptom is intrahepatic biliary obstruction with cystic dilatation of the ducts. Caroli’s disease is manifested by recurrent attacks of cholangitis, intrahepatic gallstones, and hepatic abscesses. Caroli’s syndrome rarely presents with cholangitis; its manifestations are determined by hepatic cirrhosis and portal hypertension. The risk of cholangiocarcinoma is increased 100-fold.
6.5 Acquired Diseases
6.5.1 Gallstones and Their Sequelae
Note
Gallstones are the leading cause of biliary tract disease. Frequently they are asymptomatic (“silent cholelithiasis”).
The spectrum of symptomatic cholelithiasis ranges from nonspecific upper abdominal pain and colic to life-threatening conditions such as biliary pancreatitis, gallbladder perforation, or gallstone ileus. Gallbladder carcinoma may develop as a potential late complication of chronic mechanical stone irritation.
Gallstones are caused by an imbalance in the soluble substances contained in bile. They almost always form in the gallbladder. About 15% of patients with gallbladder stones (cholecystolithiasis) also have stones in the common bile duct (choledocholithiasis).
Gallstones are classified by their chemical composition as follows:
Pure gallstones (5–10%): composed chiefly of one component of bile.
Mixed gallstones (80–90%): faceted stones made up of various components.
Combination stones (5–10%): different compositions of core and shell.
A statistical analysis of autopsy study in Leipzig from 1980 to 1989 documented the following frequency distribution: 10% pure cholesterol stones, 85% cholesterol-pigment-calcium stones, 1% pigment stones, and 3% sludge (sand). 19 For simplicity, two main types of gallstone are distinguished:
Cholesterol stones: stones composed mainly of cholesterol (75% of all gallstones).
Pigment stones: stones composed mainly of calcium bicarbonate (25% of all gallstones).
The two subtypes of pigment stones are termed brown and black stones. Cholesterol stones consist of at least 70% hardened cholesterol. They have an oval shape, while small stones are spherical. They are faceted, have a small cholesterol-pigment core, and may calcify. The formation of cholesterol stones is influenced by three factors:
Cholesterol oversaturation of bile: This results from increased hepatic cholesterol secretion (or decreased hepatic secretion of bile acids). Genetic factors play a role and involve the increased transport of cholesterol from the hepatocyte into the biliary tree (ABCG8 mutation of sterolin). 20 Other risk factors are age, obesity, rapid weight loss, female sex, pregnancy, a high-fat diet, intestinal disorders, and lipid-lowering drugs (clofibrate, etc.).
Nucleation-promoting factors: These factors include gallbladder mucus and calcium.
Impaired gallbladder emptying: This is caused by reduced gallbladder motility. Risk factors are pregnancy, rapid weight loss, and medications (contraceptives, octreotide [a somatostatin analogue]).
Pigment stones form under two conditions: Black pigment stones are associated with hemolysis or hepatic cirrhosis and are most commonly found in the gallbladder. They may also form in the intrahepatic bile ducts of a cirrhotic liver. Brown pigment stones form in association with biliary tract infections and therefore occur in the biliary tree. Pigment stones are small (1–10 mm) and usually multiple. Their color is derived from the presence of iron and copper.
Gallbladder sludge consists of small particles that precipitate from the biliary fluid. It most commonly forms in response to conditions such as rapid weight loss, consumptive illness, pregnancy, medication use (lipid-lowering drugs), and organ or bone marrow transplantation. It consistently forms in patients on prolonged parenteral nutrition (e.g., ICU patients); this underscores the importance of gallbladder emptying in the pathogenesis of lithiasis. Biliary sludge is asymptomatic in most patients. If the cause is eliminated, it may resolve. When symptoms occur, they result from duct obstruction and present as biliary colic. Like gallstones, gallbladder sludge may also cause acute cholecystitis or even biliary pancreatitis.
Silent Cholelithiasis
Brief definition Gallstones are a common occurrence, affecting approximately 10% of adults and 2% of children. Females are affected 2 to 3 times more frequently than males. There is also a familial disposition. Hepatic cirrhosis, biliary tract diseases, and infections predispose to gallstone formation. Other factors play a role in children: congenital bile duct anomalies, short bowel syndrome with impaired enterohepatic circulation, prolonged parenteral nutrition, hemolytic anemia, and cystic fibrosis. Asymptomatic stones are found almost exclusively in the gallbladder. Stones in the common bile duct will generally produce symptoms.
Imaging signs Only 20% of cholesterol stones are calcified and radiopaque (▶ Fig. 6.18).
Diagnosis of gallstones is the domain of ultrasound (▶ Fig. 6.19). A normal gallbladder is echo-free. A stone presents the following triad of sonographic features:
Curved, high-level echo with a posterior acoustic shadow.
Visualizability in two planes.
Mobility with changes in patient position.

Fig. 6.18 Cholecystolithiasis. Progression of findings in a 52-year-old man with sarcoidosis and nonspecific upper abdominal complaints without local tenderness. Antral gastritis was diagnosed by endoscopy (not shown). (a) Radiograph shows a cholesterol stone with a calcified rim in the gallbladder infundibulum (arrow). Additional small stones are present in the fundus. (b) CT scan for sarcoidosis 6 months before image [a] shows a small, stone-filled gallbladder. Here the infundibulum stone is located in the fundus (arrow).

Fig. 6.19 Gallbladder stones. Ultrasound features. (a) Large, solitary, shadowing cholesterol stone imaged in two planes (longitudinal and transverse). (b) Medium-sized stones. (c) Small, nonshadowing cholesterol stones. (d) Multiple small stones with dense acoustic shadows. Pigment stones in spherocytosis.
Small stones may not cast an acoustic shadow (see ▶ Fig. 6.18c).
Note
The size, number, and location of the stones should be noted in the radiology report.
Small stones (4–6 mm) are potentially passable. They may occlude the infundibulum or incite a choledocholithiasis leading to biliary tract obstruction or even biliary pancreatitis. A large gallstone may completely occupy the gallbladder lumen (“giant gallstone,” ▶ Fig. 6.20). Small cholesterol stones may float within the gallbladder lumen. Often they do not cast an acoustic shadow and are visible as round or polygonal bodies (see ▶ Fig. 6.19c). Gallbladder sludge appears as a mobile sediment that may be hyperechoic or isoechoic to the liver. If the gallbladder is completely filled with sludge, it may be difficult to delineate from the adjacent liver (▶ Fig. 6.21). Gallbladder sludge can mask the presence of actual stones (see ▶ Fig. 6.21d). Ultrasound is a reliable modality for detecting stones in the common bile duct, thickening of the duct wall, and intrahepatic biliary obstruction (see ▶ Fig. 6.5). Stone detection by CT is limited by the variable density of gallstones. Hyperdense calcified stones, mixed stones with a calcium component, and hypodense fatty cholesterol stones are distinguishable from biliary fluid. Approximately 20% of gallstones are isodense to the surrounding fluid and are therefore not detectable by CT; gallbladder sludge is often undetectable as well. CT is less accurate than ultrasound for evaluating the gallbladder wall (▶ Fig. 6.22). Bile is hyperintense in T2W sequences on MRI, and these sequences provide the highest sensitivity for stone detection (▶ Fig. 6.23). Fat-suppressed T1W sequences after administration of contrast medium have special importance as they can distinguish stones from gallbladder polyps based on enhancement characteristics (see ▶ Fig. 6.23d, f). MRCP is available as an adjunct to sectional imaging. MRI can detect stones as small as 2 mm in the gallbladder and biliary tree.

Fig. 6.20 Giant gallstone in an 84-year-old woman with weight loss. At surgery: chronic atrophic cholecystitis with gallbladder carcinoma and nodal metastases. (a) Giant gallstone casts a large acoustic shadow that obscures the porta hepatis and posterior gallbladder wall. (b) Scan at a more caudal level shows enlarged lymph nodes in the hepatoduodenal ligament.

Fig. 6.21 Gallbladder sludge. (a) Dependent layering of echogenic sediment. (b) Sediment layer shows a partially lobulated surface. (c) Rounded sludge mass isoechoic to liver includes small stones and a larger stone with an acoustic shadow (arrow). (d) Sludge forming an ellipsoidal cast of the gallbladder lumen (inset: transverse scan).

Fig. 6.22 Gallstones. CT shows five mixed cholesterol stones (cholesterol, pigment and calcium stones) with surface calcifications diminishing posteriorly. The interior of the stones is isodense to biliary fluid. The posterior stone is barely identifiable.

Fig. 6.23 Gallstones. MRI features. (a) Venography. (b) MR cholangiopancreatography. (c) T2W image shows a small stone in the gallbladder fundus (arrow). (d) Coronal fat-suppressed T1W image shows absence of enhancement (arrow), consistent with a stone. (e) T2W image of a small stone in the infundibulum (arrow). (f) Coronal fat-suppressed T1W image shows enhancement (arrow) consistent with a gallbladder polyp.
Clinical features Silent cholecystolithiasis remains asymptomatic in two-thirds of cases; generally it is detected incidentally at ultrasound. On the other hand, gallstones are the most frequent cause of upper abdominal pain. Patients often present with nonspecific complaints such as nausea and occasional vomiting, especially after heavy, fat-rich meals. A history of aversion to fatty, spicy foods is another classic sign.
Note
An important indicator of whether acute upper abdominal pain is due to acute cholecystitis is the tenderness of the gallbladder to pressure (Murphy’s sign). If the gallbladder is not tender, it is probably not the source of the acute upper abdominal pain.
Asymptomatic stones in an otherwise normal gallbladder do not require treatment.
Differential diagnosis
Gallbladder polyps: Polyps are not mobile in response to position changes; they are fixed to the gallbladder wall. They do not cast an acoustic shadow, and they enhance on T1-weighted MRI after IV administration of contrast medium.
Adenomyomatosis: This condition involves a segmental or diffuse wall thickening that enhances after IV administration of contrast medium.
Gallbladder tumor: Generally it is not difficult to distinguish between a stone and gallbladder tumor at ultrasound owing to the immobility of the tumor. The main criterion on CT and MRI is the occurrence of tumor enhancement. If CT or MRI findings are equivocal, the gallbladder should also be examined by ultrasound.
Key points Silent gallbladder stones can be found in 10% of adults and 2% of children. They are detected incidentally during an ultrasound examination. Approximately one-third of patients with silent stones will go on to develop symptomatic cholecystolithiasis or choledocholithiasis. The risk of symptomatic cholelithiasis depends on stone size and is greater with small, potentially passable stones. The finding of gallbladder wall changes in an asymptomatic patient is sufficient to exclude silent cholecystolithiasis. These patients should be evaluated for possible gallbladder carcinoma.
Symptomatic Cholecystolithiasis
Brief definition When gallstones migrate, they may become impacted at any level of the biliary tree and cause an obstruction. The consequent rise in pressure leads to biliary colic. Impaired bile drainage may lead to obstructive jaundice and is a risk factor for biliary tract infection. Over time the infection may ascend and reach the gallbladder. Colic may also be caused by an infundibular stone that prevents the contracting gallbladder from emptying. Stone-associated symptoms may resolve spontaneously in one-half of cases. But they may also cause potentially life-threatening complications.
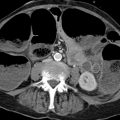
Imaging signs The gallbladder is always found to be tender on initial ultrasound scanning. Ultrasound can detect the stones and determine their size and location. Dilatation of the bile ducts may be noted depending on the site and duration of the obstruction. The normal diameter of the common bile duct is 6 mm. A value of 10 mm or more in a patient not previously operated on indicates dilatation. Stones in the gallbladder infundibulum are easily detected, whereas common duct stones are not, especially if the duct is not dilated (▶ Fig. 6.24, ▶ Fig. 6.25). If the gallbladder wall is thickened, there is cholecystitis.

Fig. 6.24 Choledocholithiasis. Ultrasound findings in an 8-year-old boy with biliary colic. (a) Stone in the dilated common bile duct. The gallbladder wall is thickened, and sludge is present in the gallbladder lumen. (b) Hypoechoic swelling (arrow) surrounds the pancreatic segment of the common bile duct in biliary head pancreatitis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree