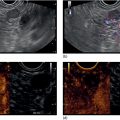
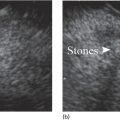
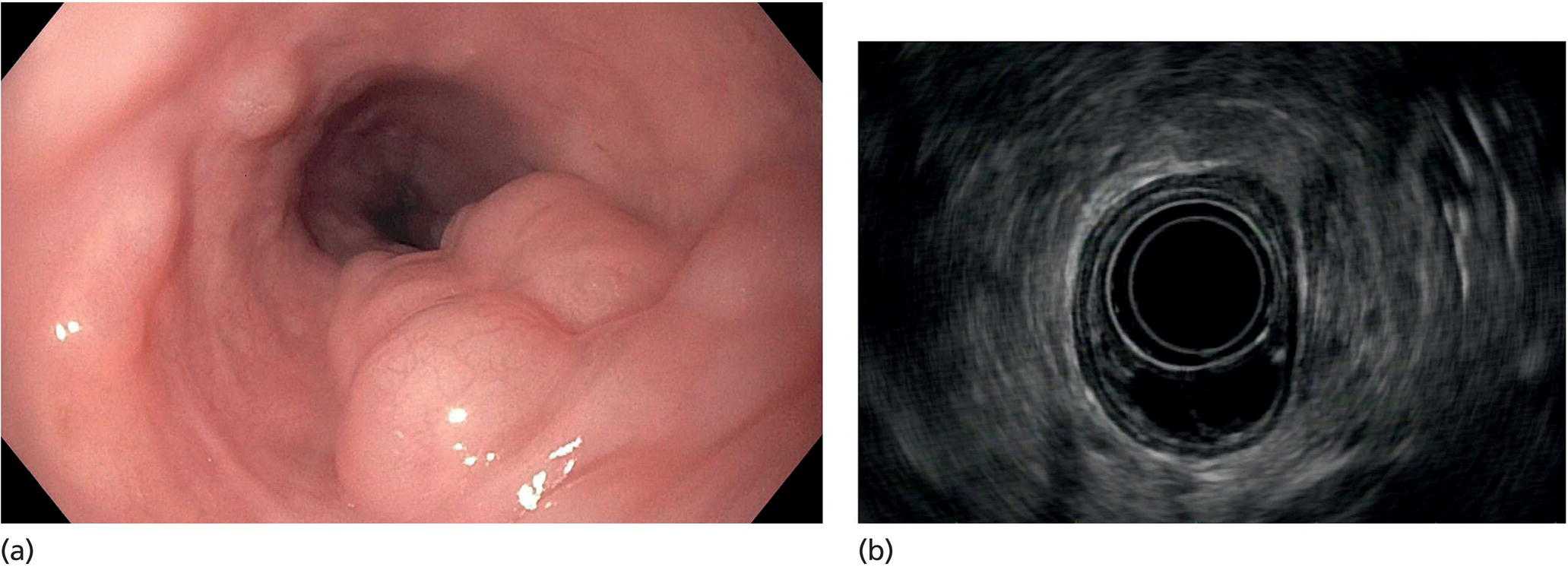
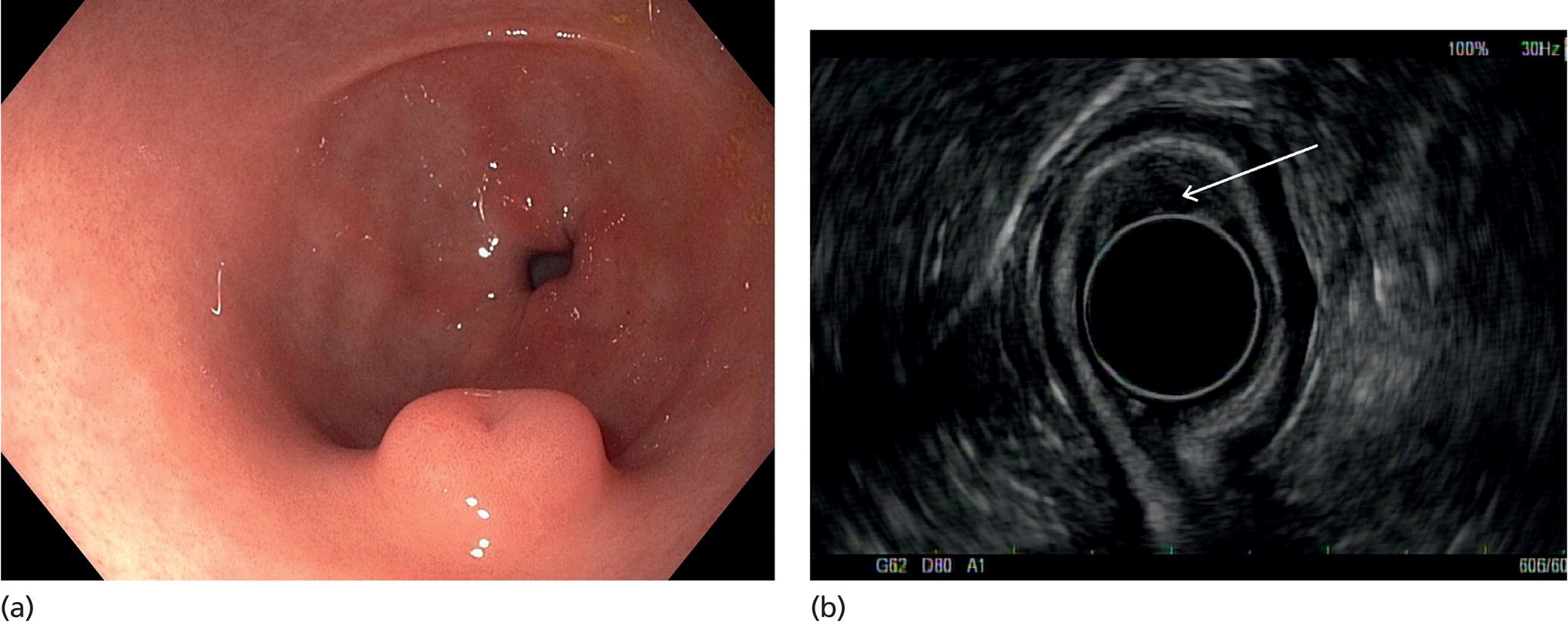
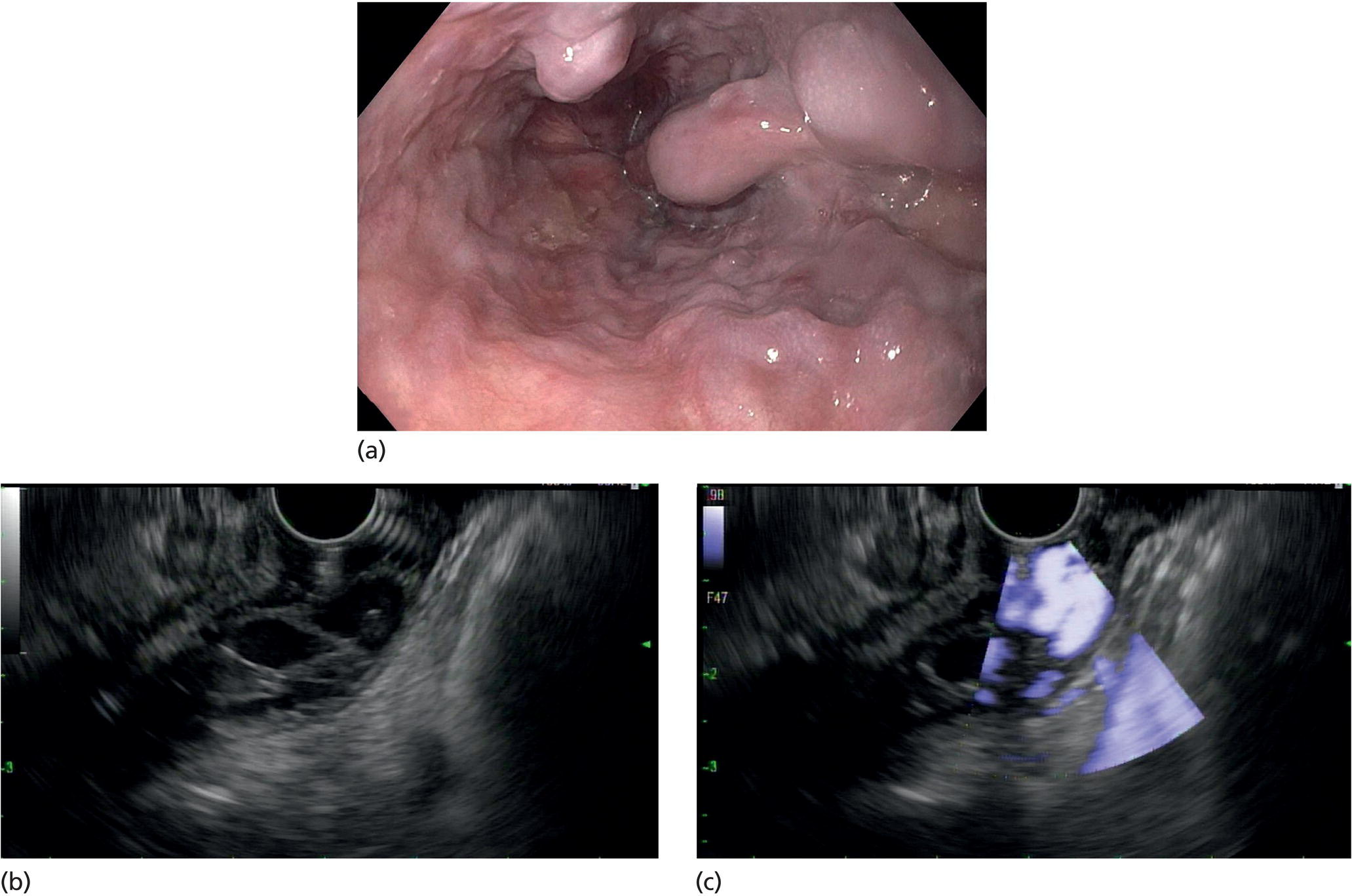
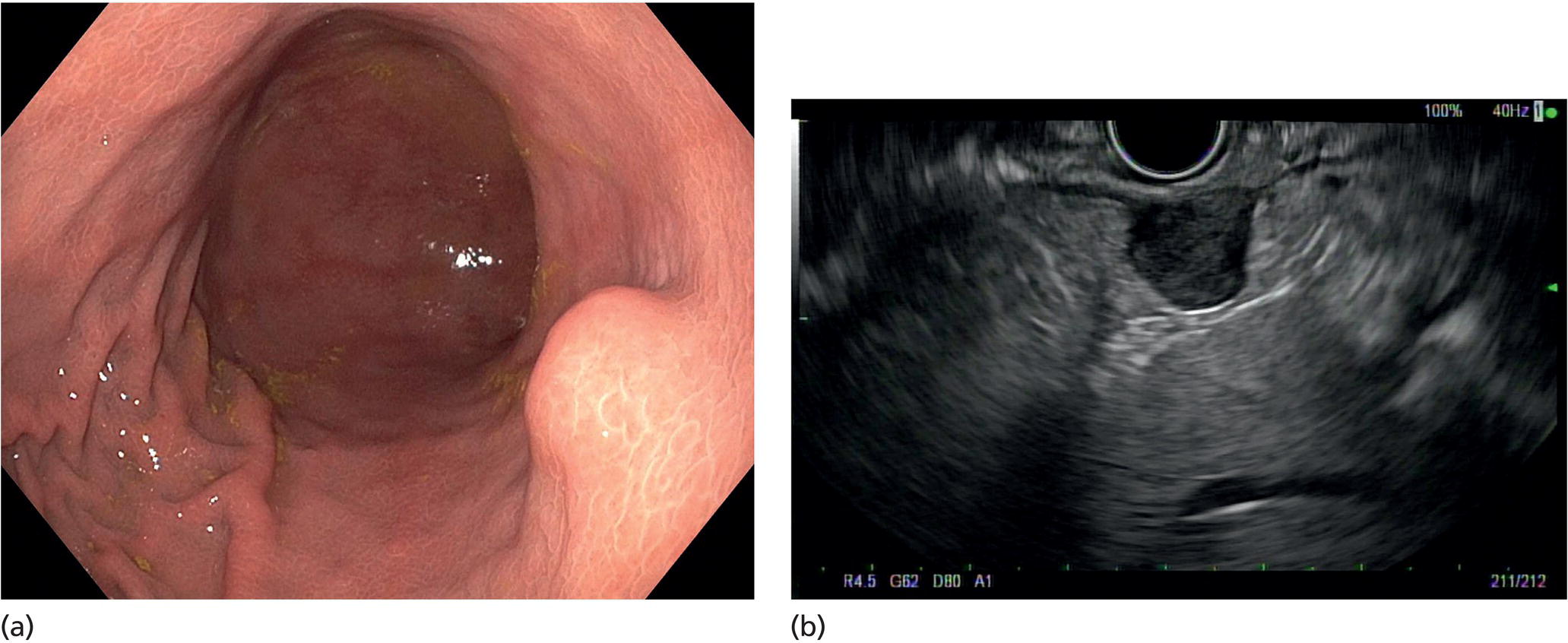
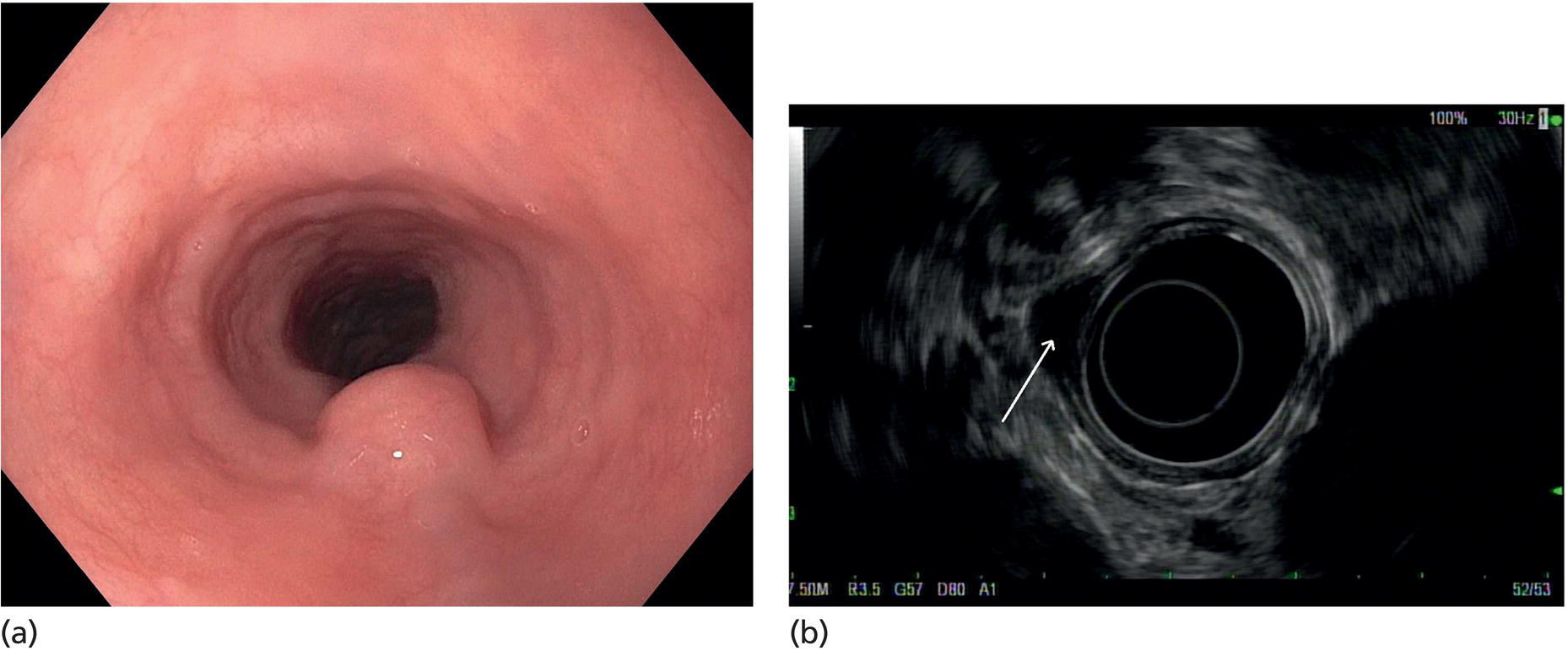
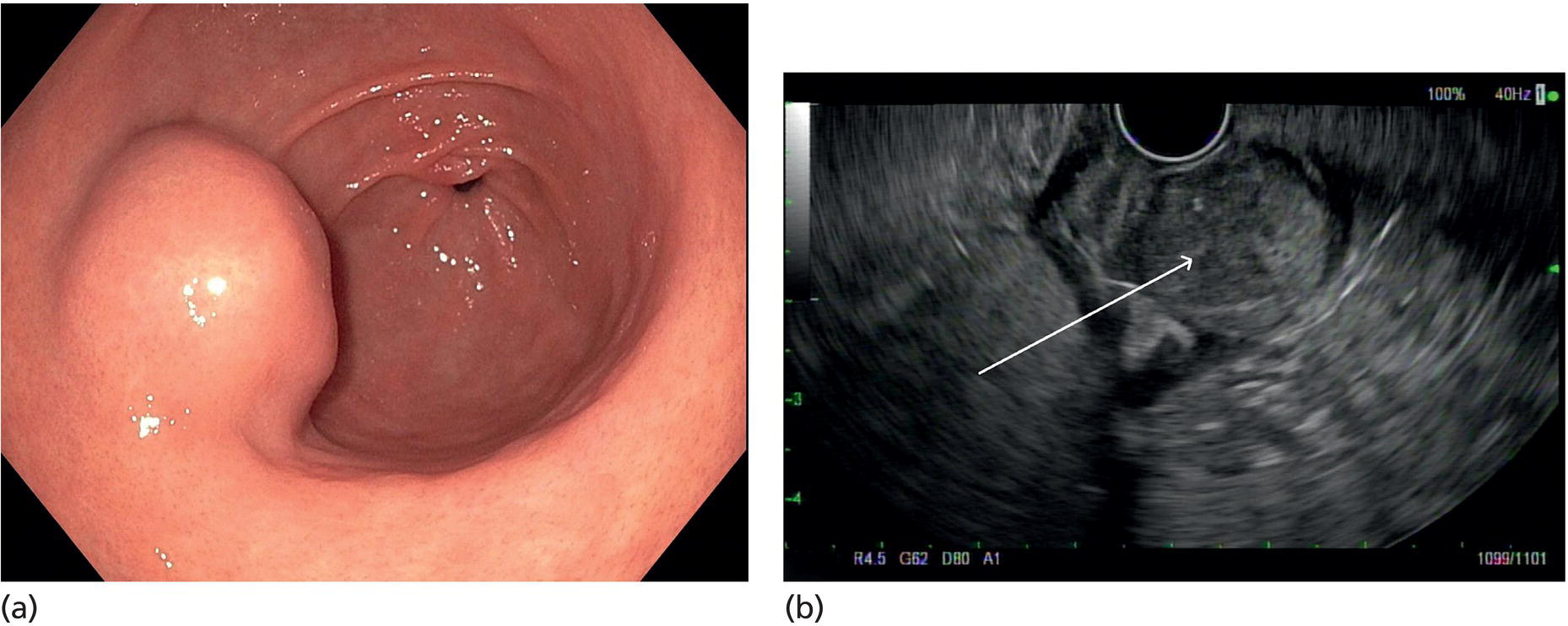
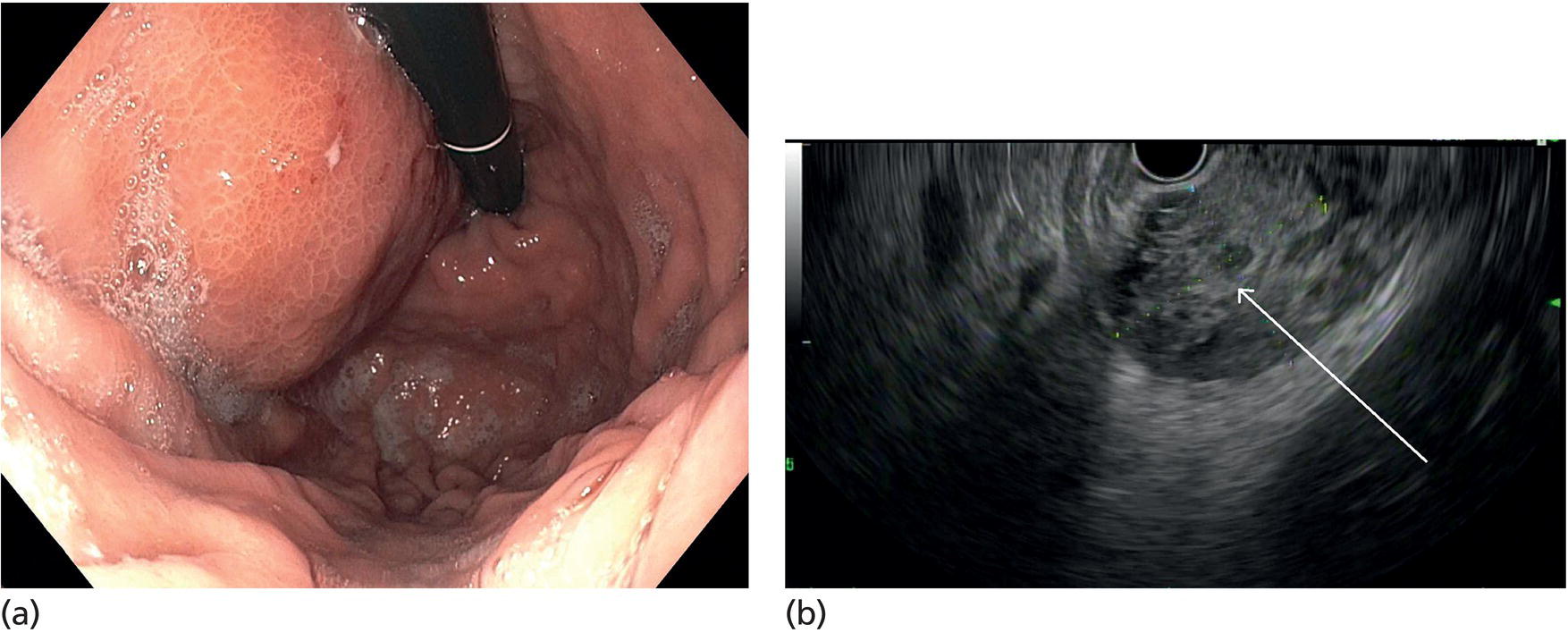
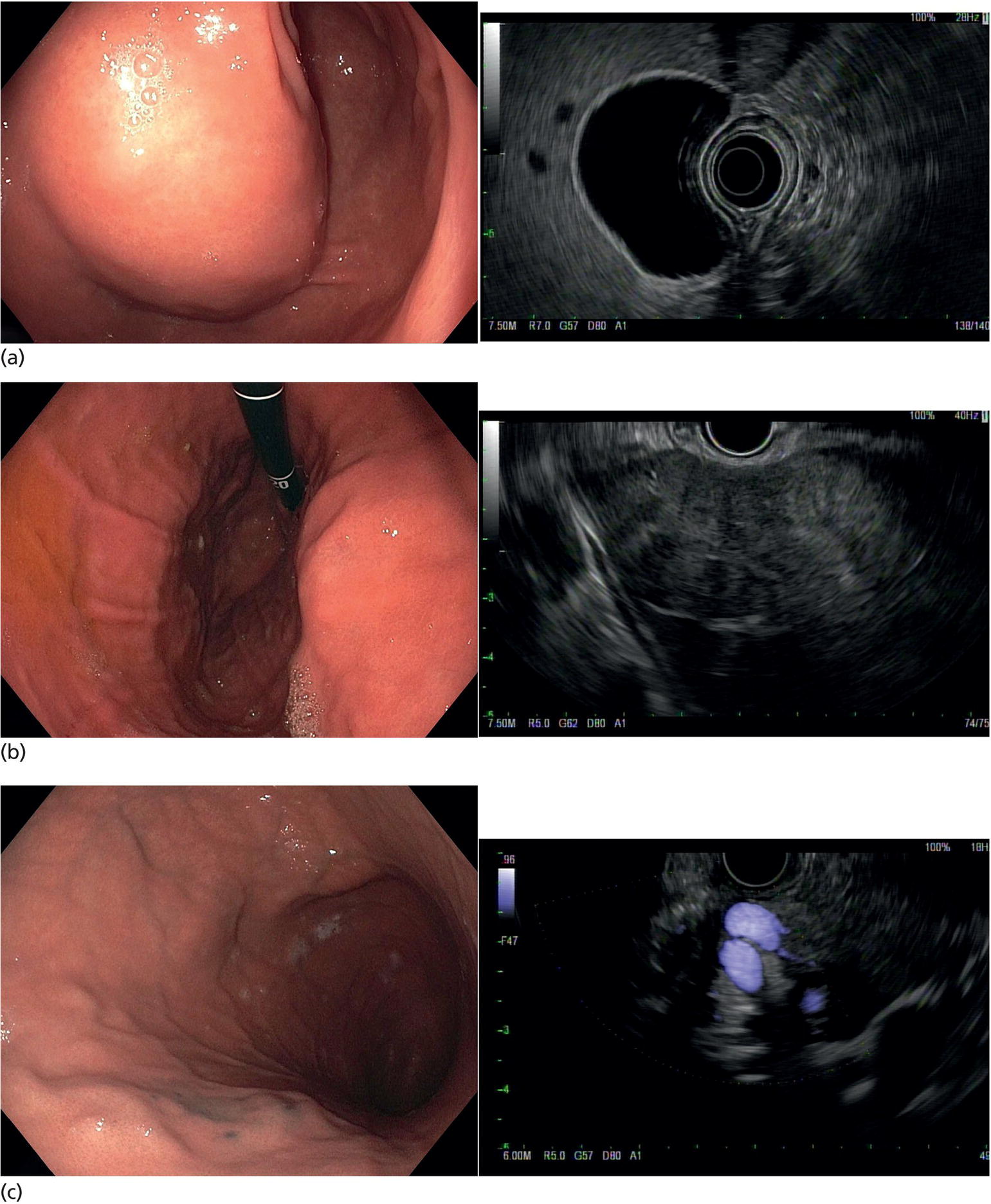
Muhammad Tahir and Andrew J. Bain Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA Gastrointestinal subepithelial masses are lesions that lie underneath normal‐appearing mucosa. They are usually asymptomatic incidental findings detected during upper endoscopy performed for other reasons. Rarely do subepithelial masses present with symptoms of bleeding or obstruction. The incidence of gastrointestinal subepithelial masses has increased with the widespread use of esophagogastroduodenoscopy (EGD) for various indications. Most esophageal and gastric subepithelial masses are benign; however, some have malignant potential. Therefore, it is important to establish a correct diagnosis and exclude malignancy. Obtaining an accurate diagnosis allows for appropriate treatment of patients with observation/reassurance versus surgical or endoscopic resection. Tissue sampling with standard endoscopic techniques is often unsuccessful in establishing a diagnosis due to inadequate depth of sampling. Endoscopic ultrasonography (EUS) is important in obtaining a diagnosis by virtue of minimally invasive tissue characterization with ultrasound, determination of wall layer of origin (Table 14.1), and by allowing fine needle aspiration (FNA) or fine needle biopsy (FNB) for pathologic diagnosis. Lipomas are benign growths of mature lipocytes that can be seen in any part of the gastrointestinal tract. Esophageal lipomas are extremely rare, whereas gastric and duodenal lipomas are more common. Endoscopically, lipomas appear as a smooth bulge with normal overlying mucosa with a discernible yellow tint (Figure 14.1a). The pillow or cushion sign, whereby a closed forceps is pressed against the mass leaving a slight indentation, is a reasonably specific endoscopic sign for lipomas. On EUS, lipomas are hyperechoic, homogeneous oval masses with smooth margins located in the submucosal layer (Figure 14.1b). Tissue sampling is rarely necessary given the excellent diagnostic accuracy of EUS characterization. If pursued, tunnel biopsies can strip away the superficial mucosa revealing glistening fat located within the submucosal layer (Figure 14.1c). Asymptomatic lipomas generally require no additional surveillance. Carcinoid tumors are neuroendocrine tumors with malignant potential. They are most commonly found in the appendix and rectum, but can also be found in the stomach, esophagus, and duodenum. Carcinoid tumors arise from the mucosal layer but can invade into the submucosal layer forming a subepithelial mass. Endoscopically, carcinoids appear as smooth, round, yellowish masses (Figure 14.2a) that can display central erythematous depression or ulceration. On EUS, carcinoids appear hypoechoic or isoechoic, homogeneous with smooth borders, and are usually located in the mucosal/submucosal layer (Figure 14.2b). Unlike most true submucosal tumors, the diagnosis of carcinoids can often be made with standard biopsy forceps. In general, endoscopic resection can be considered for carcinoid tumors of less than 1 cm located within the mucosal or submucosal layer without associated lymphadenopathy. Endoscopically resected carcinoid tumors should be followed periodically with both endoscopy and EUS to assess for local recurrence. Larger tumors should be surgically removed. Granular cell tumors (GCTs) are generally benign lesions that originate from neural tissue. They are most commonly found in the oropharynx and subcutaneous tissue, but can involve any part of the gastrointestinal tract. The most common location in the gastrointestinal tract is the esophagus followed by the stomach and colon. Although multifocal GCTs have been described, they most commonly present as solitary lesions. Endoscopically, they appear as subepithelial masses with normal overlying mucosa displaying a yellowish hue and feel firm and rubbery when palpated with biopsy forceps (Figure 14.3a). On EUS, they appear as hypoechoic lesions with smooth margins located in the mucosal and/or submucosal layer (Figure 14.3b). Endoscopic ultrasound findings that suggest malignancy include invasion through the intestinal wall and increasing size during surveillance. GCTs have a low, but not zero risk of metastasizing, and therefore attempts should be made at resection. Endoscopic mucosal resection (EMR) appears to be an excellent modality for removal of tumors less than 2 cm in diameter (Figure 14.3c–f). Surgery is most appropriate for lesions of greater than 2 cm. Repeat EGD with EUS can be used for surveillance in patients not undergoing resection. Table 14.1 Endoscopic ultrasound (EUS) characteristics of gastric and esophageal subepithelial masses. GIST, gastrointestinal stromal cell tumor. Figure 14.1 Lipoma. (a) Endoscopic view of lipoma with smooth overlying mucosa. (b) EUS showing large hyperechoic gastric mass in the submucosal layer of gastric antrum. (c) Tunnel biopsy showing glistening yellow submucosal fat tissue. Figure 14.2 Carcinoid tumor. (a) Endoscopic view of a carcinoid tumor. (b) Endosonographic view of a carcinoid tumor which appears as an oval homogeneous, hypoechoic mass located in the submucosa. Figure 14.3 Granular cell tumor. (a) Endoscopic view of esophageal granular cell tumor. (b) EUS showing a 6 × 3 mm well‐defined hypoechoic mass located within the submucosal layer of the esophageal wall. (c) Band ligation EMR of a granular cell tumor with band in place (d) Band ligation EMR of a granular cell tumor with snare below the band. (e) Granular cell tumor EMR defect. (f) Granular cell tumor EMR specimen. Duplication cysts arise during embryonic development, estimated to occur in 1 in 8000 live births. These cysts can be located anywhere within or adjacent to the luminal gastrointestinal tract and the majority do not communicate with the lumen. They are spherical or tubular, contain mucin, possess a smooth muscle layer, and are lined by the same mucosa as the adjacent bowel. It is not uncommon for esophageal duplication cysts to be multifocal (Figure 14.4a). Duplication cysts are usually asymptomatic, but can result in symptoms due to mass effect, bleeding, or perforation. Duplication cysts rarely have been reported to undergo malignant transformation. Endoscopic ultrasound of duplication cysts usually reveals a round anechoic lesion in the submucosa that has acoustic enhancement (increased through transmission of ultrasound signal which gives a bright appearance on the side distal to the ultrasound transducer, similar to a flashlight effect) (Figure 14.4b). They may have endosonographic findings of echogenic debris which can be mucinous material. The diagnosis can be confirmed with FNA of the fluid, although this is not generally necessary and may unnecessarily expose the patient to risk of cyst infection. The optimal management of asymptomatic cysts is unknown. Periodic EUS surveillance to observe for enlargement may be appropriate. Treatment of symptomatic or enlarging duplication cysts has traditionally been surgical resection. Pancreatic rest (also known as aberrant pancreas, ectopic pancreas, and heterotopic pancreas) are submucosal tumors consisting of dilated exocrine cells. The majority are 10 mm in diameter, located 1–4 cm from the pylorus in the 6 o’clock endoscopic position in the gastric antrum (Video 14.1). Endoscopically, they appear as submucosal masses with smooth overlying mucosa and may display characteristic central umbilication through which secretions drain (Figure 14.5a). On EUS, pancreatic rests are hypoechoic and/or heterogeneous lesions located in the mucosal/submucosal layer (Figure 14.5b). Occasionally, EUS can visualize a ductal structure traveling upward to the mucosal surface. Asymptomatic pancreatic rests require no further evaluation or treatment; however, those that are symptomatic, enlarging, or in which the diagnosis is in question should be removed endoscopically or surgically. The surgical resection is preferred when muscularis propria is involved. Figure 14.4 Esophageal duplication cysts. (a) Endoscopic view of several esophageal duplication cysts. (b) EUS showing a 13 × 6 mm anechoic lesion in the submucosal layer of the distal esophageal wall. Gastroesophageal varices can occur within the submucosal layer (esophageal or gastric varices) or outside the wall as collateral vessels (paraesophageal or paragastric varices) (Figure 14.6a). The EUS appearance is that of tubular anechoic structures that can be traced with the ultrasound probe (Figure 14.6b). Doppler ultrasound allows for easy visualization of varices (14.6c). For detection of esophageal varices, endoscopy is more sensitive than EUS, but for gastric varices EUS is more sensitive than endoscopy. Spindle cell neoplasms are the most common mesenchymal tumors of the gastrointestinal tract. These include benign leiomyomas, as well as gastrointestinal stromal cell tumors (GISTs) which have some malignant potential. GISTs are thought to originate from the interstitial cells of Cajal. The majority (90%) of GISTs are c‐kit positive, which confers a gain of function resulting in cell proliferation and survival. Approximately two‐thirds of all GISTs occur in the stomach. Endoscopy reveals a submucosal mass which is often dumbbell‐shaped and may have mucosal ulceration (Figure 14.7a). Clinically they may present as the source of upper GI bleeding, or as an incidental finding. The EUS appearance of GISTs is usually a hypoechoic mass that originates from the muscularis propria (Figure 14.7b), although occasionally they can arise from the muscularis mucosa. The EUS features associated with risk of metastases for GISTs include a tumor size greater than 4 cm, irregular extraluminal border, echogenic foci (>3 mm), and cystic spaces (>4 mm). Endoscopic and endosonographic imaging cannot differentiate benign leiomyomas (Figure 14.8) from potentially malignant GISTs. Therefore, EUS‐FNA or FNB is commonly performed to obtain tissue for immunohistochemical staining for c‐kit. The majority of GISTs are c‐kit positive, whereas leiomyomas are c‐kit negative. EUS‐guided fine needle biopsy with newer core needles often provides sufficient material to comment on the number of mitoses per high power field, which is a marker for metastatic potential. For this reason, EUS‐FNB may be preferred over traditional FNA. Surgical resection is the treatment of choice for symptomatic or large GISTs, although tumors smaller than 3 cm without EUS features of malignancy can be followed serially with EUS. Metastastic disease is treated with imatinib, a selective inhibitor of certain tyrosine kinases including c‐kit. Figure 14.5 Pancreatic rest. (a) Endoscopic view of a gastric antral pancreatic rest. (b) EUS showed a 9 × 4 mm hypoechoic, heterogeneous mass located within the submucosa of the gastric antrum. Figure 14.6 Esophageal varices. (a) Endoscopic view of grade 3 esophageal varices. (b) Esophageal varices on EUS without Doppler. (c) Esophageal varices on EUS with Doppler. Glomus tumors are extremely rare mesenchymal tumors composed of modified smooth muscle cells representing a neoplastic counterpart of the perivascular glomus bodies. They are most commonly found in the skin, but can also be found in the gastrointestinal tract, with the gastric antrum representing the most common location. Endoscopically, they appear as subepithelial masses with smooth overlying mucosa (Figure 14.9a). On EUS, they typically originate from the muscularis propria (Figure 14.9b). Glomus tumors can appear hyperechoic or hypoechoic and tend to have internal hyperechoic foci. They also manifest a strong Doppler signal consistent with the vascular nature of these tumors. EUS‐FNA immunostaining helps to differentiate glomus tumors from GISTs and carcinoid tumors. Treatment usually is surgical excision. Gastritis cystica profunda (GCP) is a benign tumor that is usually composed of gastric glands. The pathogenesis is unclear, although ischemia and chronic inflammation are thought to be the most important factors. The most common location is in the body and antrum. The endoscopic appearance varies from prominent gastric folds to a protruding subepithelial mass (Figure 14.10a). On EUS, GCP usually appears as a heterogeneously enhancing lesion with cystic spaces within the submucosal layer (Figure 14.10b). Figure 14.7 Gastric gastrointestinal stromal tumor (GIST). (a) Endoscopic view of a gastric body GIST. (b) EUS view of a GIST appearing as a 16 × 11 mm hypoechoic well‐defined mass originating from the muscularis propria of the gastric body. Figure 14.8 Leiomyoma. (a) Endoscopic view of an esophageal leiomyoma. (b) EUS shows hypoechoic well‐defined mass originating from the esophageal muscularis propria. Figure 14.9 Glomus tumor. (a) Endoscopic view of a gastric antral glomus tumor. (b) EUS showing a 20 × 18 mm isoechoic well‐defined mass that appeared to originate from the gastric muscularis propria. Figure 14.10 Gastritis cystica profunda (GCP). (a) Endoscopic view showing GCP in the fundus. (b) EUS showing a heterogeneous submucosal mass with cystic spaces. Normal abdominal organs can cause indentations in the gastrointestinal tract that may mimic a submucosal mass (Figure 14.11). Extrinsic compression can be seen in the esophagus by the aortic arch, left atrium, and spine and in the stomach by the gallbladder (Figure 14.11a), the left lobe of the liver (Figure 14.11b), and splenic vessels (Figure 14.11c). Pathologic compression may occur from malignant lymph nodes or masses in either the stomach or the esophagus. EUS can identify lesions outside the gastrointestinal tract exerting mass effect on the intestinal lumen. Table 14.2 summarizes the different lesions with the corresponding intraluminal location. Figure 14.11 Extrinsic compression. (a) Endoscopic and EUS view of extrinsic compression from the gallbladder. (b) Endoscopic and EUS view of extrinsic compression from the left lobe of the liver. (c) Endoscopic and EUS Doppler view of extrinsic compression from the splenic vessels. Table 14.2 Extrinsic compression of the esophagus and stomach.
14
Gastric and Esophageal Subepithelial Masses
Introduction
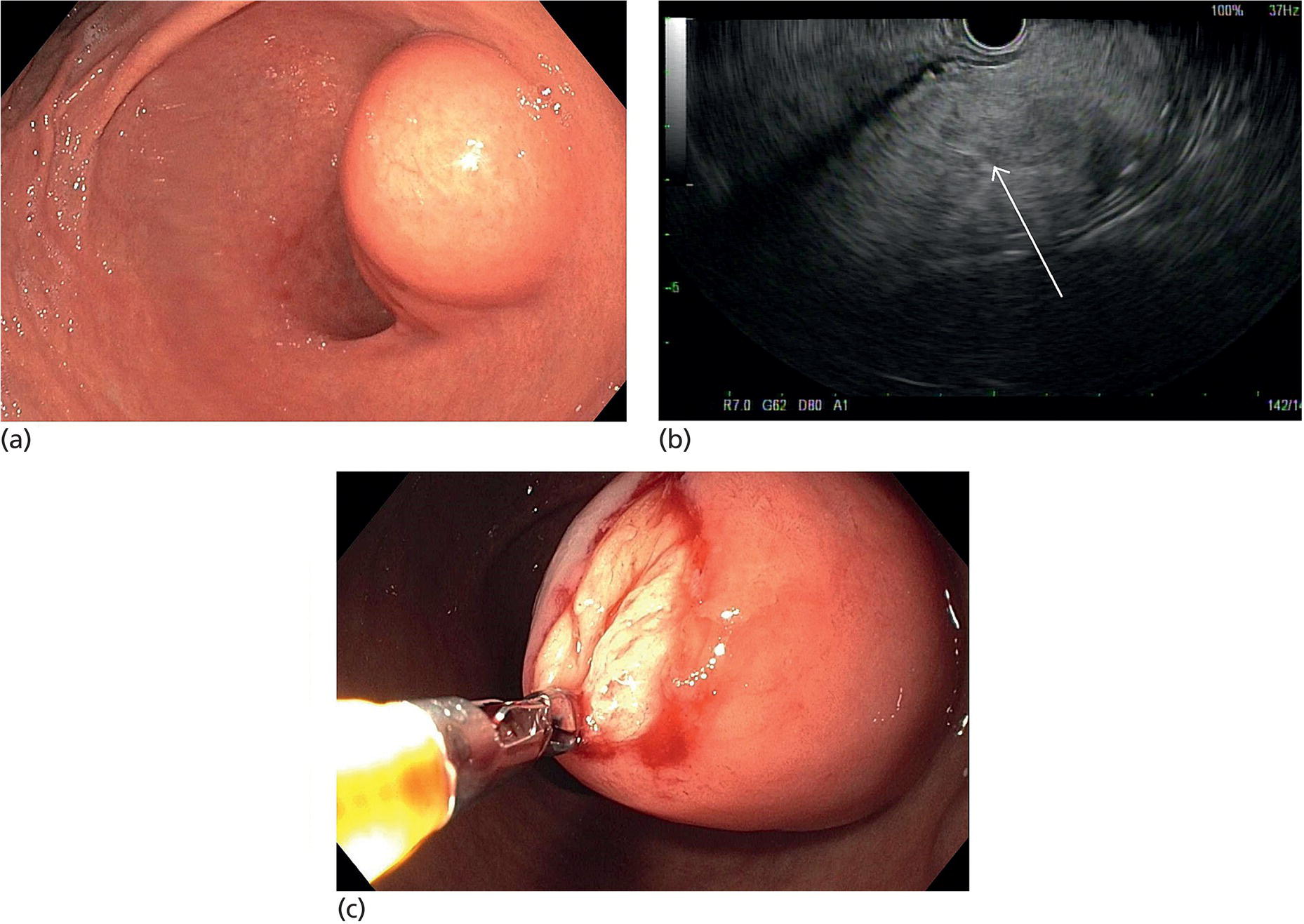
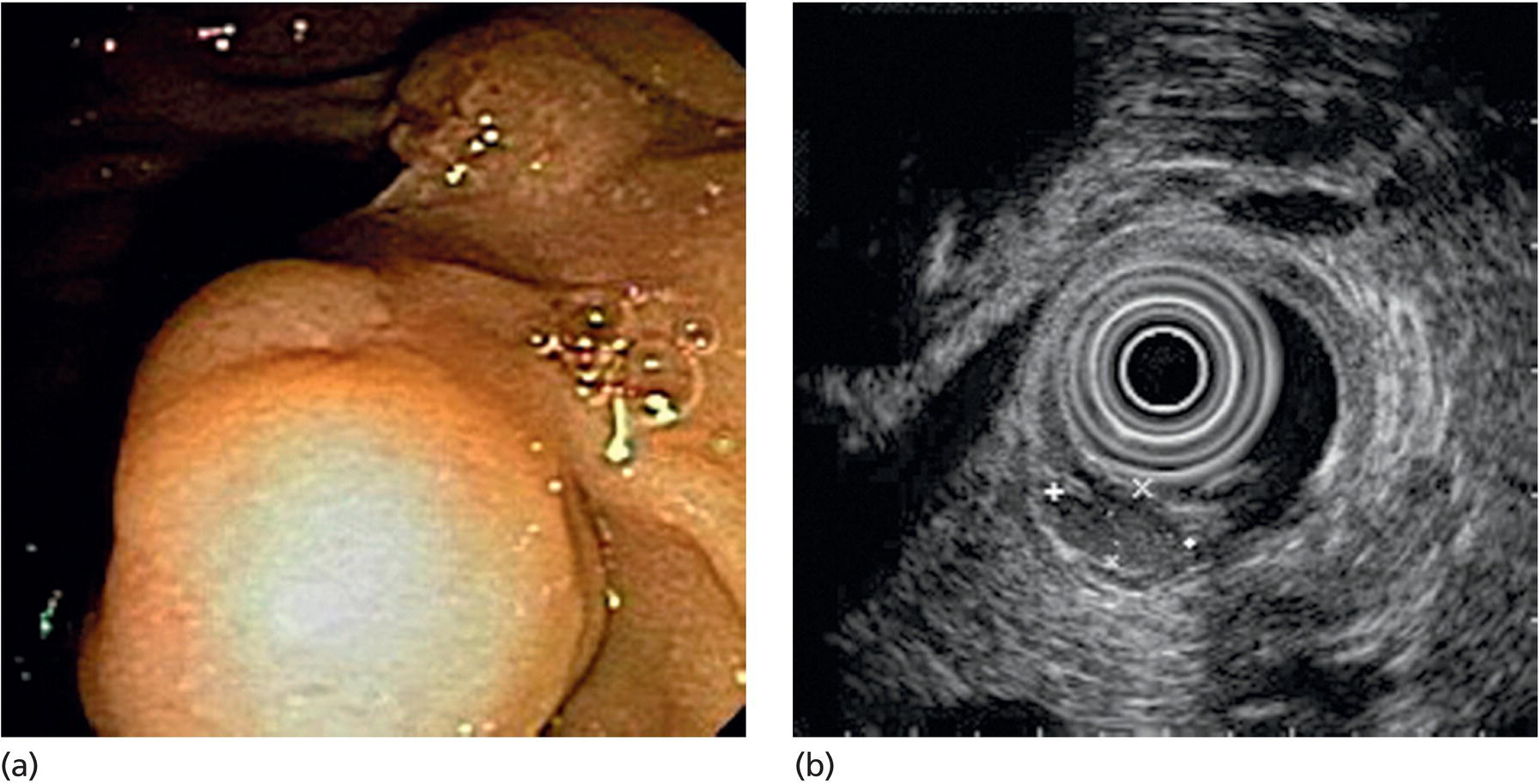
Lipoma
Carcinoid tumors
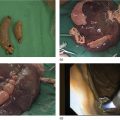
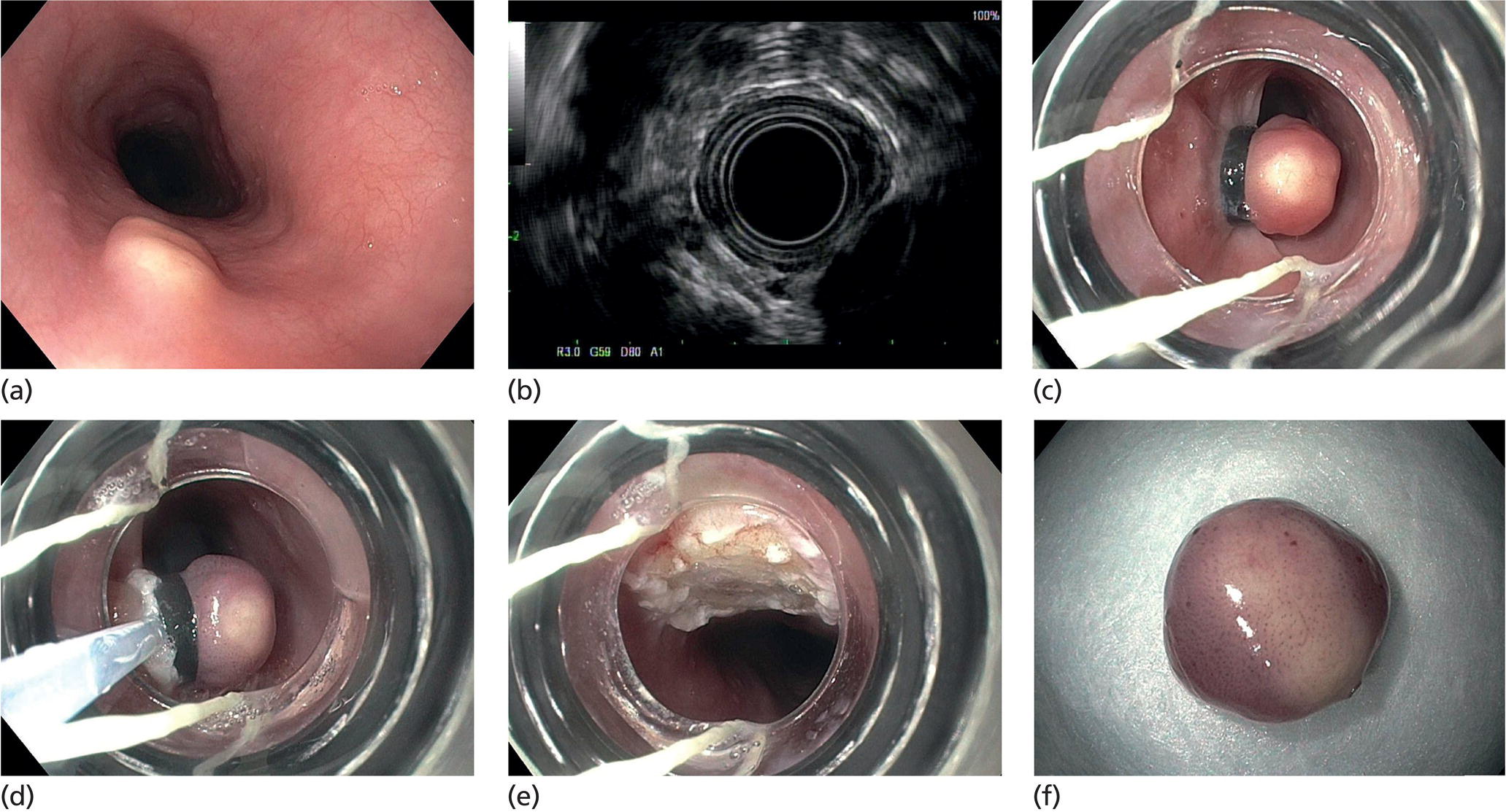
Granular cell tumor
Lesion
Wall layer
EUS features
Lipoma
Submucosa
Hyperechoic, homogeneous, oval
Carcinoid tumor
Mucosa/submucosa
Hypoechoic/isoechoic, homogeneous
Granular cell tumor
Submucosa
Hypoechoic
Duplication cyst
Submucosa
Anechoic, round, acoustic enhancement
Pancreatic rest
Submucosa/mucosa
Hypoechoic, heterogeneous, ductal structure
Varices
Submucosa
Anechoic, tubular, Doppler flow
GIST and leiomyoma
Muscularis propria
Hypoechoic
Glomus tumor
Muscularis propria
Hyperechoic/hypoechoic with strong Doppler signal
Gastritis cystica profunda
Submucosa
Heterogeneous, cystic spaces
Extrinsic compression
Outside of wall layers
Lesion dependent
Duplication cyst
Pancreatic rest
Varices
Gastrointestinal stromal cell tumors and leiomyomas
Glomus tumor
Gastritis cystica profunda
Extrinsic compression lesions
Type of extramural lesion
Most common location of intraluminal impression
Aortic arch, dilated left atrium
Esophagus
Mediastinal lymph node and tumors
Esophagus
Gallbladder
Gastric antrum
Left lobe of the liver
Proximal gastric body
Splenic vessels
Proximal gastric body, posterior wall
Pancreatic lesions
Gastric body or antrum, posterior wall
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree