To the radiologist who cares for adults, the exact age of a patient is rarely of great importance. To be sure, one approaches the chest radiograph of a 30-year-old differently from that of an 80-year-old. For the most part, however, we are all equally prey to the afflictions of senescence. For the pediatric radiologist, the situation is entirely different. To offer an interpretation of an image of a child without knowing the precise patient age is often an exercise in futility. This is especially true in the field that is the subject of this chapter: pediatric gastrointestinal (GI) imaging. To give just one example out of many that are possible, there is essentially no overlap in the differential diagnosis of small-bowel obstruction in children who are 1 day, 1 week, 1 month, or 1 year old. As discussed later in this chapter, it is an excellent rule of thumb that in any child between 3 months and 3 years of age, a small-bowel obstruction is due to intussusception until proven otherwise. Intussusception, however, almost never occurs in a newborn. In pediatric GI radiology, it is only a slight exaggeration to say that to know the patient’s age is to know the patient’s diagnosis.
It is for this reason that this chapter, devoted to imaging of the GI tract, is organized by age and not by the perhaps more familiar anatomic or pathologic categories. We believe it is more practical to present the GI radiology of the newborn than to organize a discussion around “developmental anomalies” or “diseases of the colon.”
This chapter will focus primarily on conventional radiography and fluoroscopy. The interpretation and, in the case of fluoroscopy, the performance of these studies are sometimes described as dying arts. It is probably true that the radiology resident is often most uncomfortable with these more traditional modalities. It is hoped that this chapter will prove them to be as indispensable as ever.
Imaging Techniques
Conventional Radiography
In a child with abdominal complaints, conventional radiographs are most often the recommended initial imaging evaluation. Although the referring clinician may request ultrasound or computed tomography (CT) as the first-line study in a diagnostic workup, radiographs may provide useful information to best tailor cross-sectional imaging to a patient’s specific problem.
A two-view study is most often obtained for children with acute abdominal symptoms, with supine and upright projections. In babies and patients who are very ill or uncooperative, a left lateral decubitus view may replace the upright image. An additional prone image can be useful when obstruction cannot be excluded with standard views. Air will rise to the rectum in the prone position when obstruction is not present. A single supine view alone will suffice in certain clinical settings, such as the evaluation for constipation, the search for an ingested foreign body, or the localization of catheter placement.
Normal Bowel Gas Pattern
In the newborn, air is typically present in the stomach at birth. By 6 hours of life, the stomach and the majority of the small bowel are usually filled with air. Air should reach the rectum by 24 hours of age, although this most often occurs much earlier. Loops of gas-filled bowel should be uniformly distributed throughout the abdomen and pelvis in a so-called polygonal pattern [ Fig. 4.1A ].

Abnormal Bowel Gas Patterns
Absence of air in the stomach by 1 hour of life should raise concern for an esophageal obstruction, as may be seen in the setting of esophageal atresia (EA). The presence of a “double-bubble” sign [ Fig. 4.1B ], reflecting distention of the stomach and proximal duodenum, raises concern for an upper GI obstruction. The presence of many dilated bowel loops on a neonatal abdominal radiograph raises concern for a distal bowel obstruction [ Fig. 4.1C ]. Neonatal bowel obstruction will be covered extensively in subsequent sections of this chapter.
Adynamic ileus may be seen in infants and older children. This commonly occurs after surgery and may also be seen in the setting of an acute illness such as gastroenteritis. Radiographs typically show distention of small bowel, with air–fluid levels noted on the upright or decubitus view.
With dynamic ileus, there is an anatomic cause for mechanical obstruction. Relatively common causes beyond the neonatal period include inflammation related to acute appendicitis, intussusception [ Fig. 4.2 ], inguinal hernia, postoperative adhesions, and midgut volvulus.

Abdominal Masses
An abdominal or pelvic mass may be first detected on radiographs [ Fig. 4.3 ]. An abnormal soft tissue opacity may be seen, or a solid visceral contour may be enlarged, distorted, or ill-defined in the presence of a mass. Associated calcification(s) may be present. Bowel loops may be compressed or significantly displaced by a mass, and a secondary bowel obstruction can be seen.

Pneumoperitoneum
Free air in the abdomen most often results from perforation of a hollow viscus. It is also commonly observed in the postoperative setting. Small amounts of free air may be seen on upright and decubitus views, but may be difficult to detect on a supine view. Large amounts of free air are typically readily identifiable on a supine radiograph [ Fig. 4.4 ]. A “Rigler sign” may be seen, with air on both sides of the bowel wall. A “football sign” may also be noted, with free air outlining the falciform ligament.

Ascites
Ascites has many causes in the pediatric population, as in adults. In the neonate, urinary ascites may be seen in the setting of posterior urethral valves, with obstruction of the collecting system and resulting forniceal rupture. Peritonitis may be seen after bowel perforation in the setting of meconium ileus or necrotizing enterocolitis (NEC). Chylous ascites may result from birth trauma and may also be seen in the postoperative setting.
Regardless of cause, ascites appears similar on radiographs. Bowel loops are centralized on the supine view, the hepatic margin may be indistinct, and the properitoneal fat planes may be obliterated by free fluid in the paracolic gutters. Notably, ultrasound and CT are more sensitive modalities for the detection of small amounts of free fluid in the abdomen.
Calcifications
Calcifications are well visualized on abdominal radiographs. Sheetlike calcifications may be present along the peritoneal cavity in a newborn with meconium peritonitis [ Fig. 4.5 ], or a meconium pseudocyst may be seen [ Fig. 4.6 ]. Calcification of the adrenal gland(s) related to neonatal hemorrhage may be noted. Renal calculi, gallbladder calculi, and appendicoliths are often detected on radiographs. Calcified tumors may be first suggested on radiographs, as indicated earlier.


Fluoroscopy
Contrast Agents
Barium is routinely used in the evaluation of the pediatric GI tract, most commonly for the upper GI series. Barium compounds are less frequently used for evaluation of the lower GI tract. They are relatively thick and tenacious, and often are difficult to evacuate from the colon after a contrast enema. A relative contraindication to the use of barium is suspected bowel perforation. Barium in the peritoneal cavity or retroperitoneum may result in peritonitis, granuloma formation, and adhesions.
Several water-soluble contrast agents exist. Diatrizoate meglumine/sodium (Gastrografin) is a hyperosmolar water-soluble contrast medium. It is never used in the routine diagnostic evaluation of the upper GI tract. There is a serious risk for pulmonary edema or death with aspiration because aspirated Gastrografin can trigger the release of histamine or histamine-like substances in the lung. Hyperosmolar, hydrophilic contrast agents like Gastrografin also draw fluid into the GI tract lumen and can result in massive and potentially dangerous fluid shifts. This is particularly true in neonates. If ingested from above, marked dilution of hyperosmolar agents occurs as early as the third portion of the duodenum, limiting their diagnostic utility for the remainder of the GI tract. The hyperosmolar quality of these agents may be exploited in the large bowel in certain clinical settings (to be described). Through appropriate dilution of these contrast agents, near isotonicity can be achieved.
Iothalamate meglumine (Cysto-Conray) is less hypertonic than agents like Gastrografin. The relatively low iodine content of this contrast medium limits adequate visualization of detail for upper GI studies. However, Cysto-Conray does provide adequate visualization of the lower GI tract and is the agent of choice for routine diagnostic contrast enemas.
Water-soluble agents with relatively lower osmolality include iopamidol (Isovue) , ioversol (Optiray) , and iohexol (Omnipaque) . These agents contain sufficient iodine concentration for adequate visualization of the upper GI tract but are low enough in osmolality to prevent clinically significant fluid shifts. They are useful when the anatomic integrity of the GI tract must be evaluated in the sick neonate, as in the setting of NEC. They are also useful in the postoperative setting after bowel anastomosis, when the risk for perforation is relatively high. Notably, use of these agents is best reserved for these and other specific circumstances. They are more costly and often less revealing than more radiopaque barium.
Common Examinations
The upper GI series is routinely performed in the pediatric population. Common indications include the assessment for upper GI obstruction in a neonate, malrotation of the bowel with or without midgut volvulus, and anatomic malformations such as tracheoesophageal fistula (TEF). These and other entities will be discussed in detail in later sections of this chapter.
Pediatric patients most often ingest dilute barium alone for an upper GI. The swallowing mechanism is assessed, and evaluation for laryngeal penetration and tracheal aspiration is performed. The contour and motility pattern of the esophagus and the gastroesophageal (GE) junction are evaluated, and the anatomic integrity of the stomach, duodenum, and proximal jejunum is assessed. Most importantly, the location of the duodenojejunal junction (DJJ) is determined to assess for normal rotation and positioning of the GI tract. Normally, the DJJ is positioned to the left of the spine posterior to the stomach at the level of the duodenal bulb [ Fig. 4.7 ]. A normal DJJ may be located a bit medial to this overlying a left-sided spinous pedicle, or slightly lower than the level of the duodenal bulb. The duodenal C-sweep is usually smooth but may demonstrate some degree of undulation or redundancy. These represent variations of normal; the position of the DJJ itself is of paramount importance.
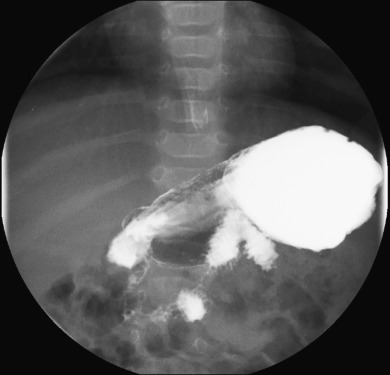
Double-contrast studies are worthwhile for the evaluation of the GI tract mucosa. However, these studies are infrequently indicated in the pediatric population and not typically believed to be high yield. The contrast enema is another routine fluoroscopic examination in the pediatric population. This study is most commonly performed to evaluate for low bowel obstruction in the neonate, as may be seen in Hirschsprung disease, small left colon syndrome, meconium ileus, and ileal atresia.
As indicated earlier, water-soluble contrast agents such as iothalamate meglumine (Cysto-Conray) are most appropriate for a diagnostic contrast enema.
The contrast enema is typically performed with a small, straight-tipped catheter inserted low in the rectum so that distal pathology is not missed. Contrast is instilled throughout the large bowel, ideally with reflux to the distal ileum. The caliber and contour of the colon are assessed, and evaluation for areas of narrowing or obstruction is performed.
Occasionally a therapeutic enema may be performed. Indications include the therapeutic contrast enema performed for meconium ileus in the newborn with cystic fibrosis (CF) and distal intestinal obstruction syndrome (DIOS) in the older patient with CF. The contrast agent of choice for these studies is diatrizoate meglumine/sodium (Gastrografin). The high osmolality of Gastrografin causes fluid shifts into the GI tract lumen, with the potential to relieve obstruction related to tenacious meconium or significant stool impaction.
Another commonly performed therapeutic enema in the pediatric population is the air enema for intussusception reduction. This procedure is outlined in detail later in this chapter.
The Newborn
Intestinal Obstruction in the Newborn
Intestinal obstruction is the most common abdominal emergency in the newborn period. Almost all babies with the conditions to be described will present in the first day or two of life. The single exception is malrotation with midgut volvulus. Even with this condition, however, babies will usually develop symptoms within the first month of life.
Neonatal bowel obstruction is usually classified as high or low [ Box 4.1 ]. High or proximal obstruction involves the stomach, duodenum, jejunum, and proximal ileum; low or distal obstruction involves the distal ileum and colon. Obviously there is no precise location after which a “high” obstruction becomes “low.” By the end of this section, however, it should be clear why such a seemingly vague classification is not only necessary but also extraordinarily useful.
The presentation of both types of obstruction may be identical. Babies can present with vomiting that is often bilious, abdominal distention, delayed or absent passage of meconium, or poor feeding. Fortunately, the distinction between high and low obstruction can almost always be made from a single abdominal radiograph. Air is an excellent contrast medium. Air is swallowed at birth and either passes out the other end of the GI tract or stops at the point of obstruction. Neonates with a high obstruction will have one, two, or a few air-filled dilated bowel loops, and those with a low obstruction will have many [ Fig. 4.1 ]. This distinction is critical because virtually all babies with a high obstruction need surgery. Babies with low obstruction initially need a contrast enema. The enema will usually yield a specific diagnosis and, in some cases, may be therapeutic.
Low Intestinal Obstruction
As stated earlier, all newborns with signs and symptoms of intestinal obstruction need an abdominal radiograph. To the request frequently made of the radiologist, “We have a newborn with bilious vomiting and would like an upper GI series to rule out malrotation,” the appropriate response is, “Let’s start with a plain radiograph. After that, we will decide which study is indicated.” If the radiograph demonstrates multiple dilated bowel loops, the baby may have a low or distal obstruction and needs an enema, not an upper GI. The exact level of the obstruction, whether in the distal small bowel or the colon, cannot be determined from the radiograph alone. The contrast enema will be definitive.
The enema should be performed with water-soluble contrast material. We use a relatively dilute ionic agent such as that used for cystography. There is no need for more expensive nonionic agents or for barium because we are rarely interested in mucosal detail in newborns. Babies with meconium plug/small left colon syndrome or meconium ileus (discussed later) may actually benefit from a water-soluble enema. Further, in our experience, the use of barium as a diagnostic agent actually decreases the chance of subsequent successful therapeutic reduction of meconium ileus. The diagnostic enema should be performed with a small-caliber tube barely inserted into the rectum so that very distal pathology is not missed.
The differential diagnosis of low obstruction is limited [ Box 4.1 ]. Three conditions involve the colon: Hirschsprung disease, small left colon/meconium plug syndrome, and colonic atresia. Two conditions involve the distal ileum: meconium ileus and ileal atresia. A radiologist may spend many years in a busy pediatric hospital and see only these conditions.
Anorectal malformations such as imperforate anus may, of course, cause low obstruction, although the diagnosis should be clinically obvious. Preoperative imaging in these patients is usually unnecessary. Anorectal malformations are discussed at the end of this section.
The crucial differential diagnostic finding on the contrast enema is the presence or absence of a microcolon. A microcolon is simply a colon of very small caliber. The entire colon must be small, not just a part of it. The assessment of the colon as “small” or “micro” is necessarily subjective and requires some experience [ Fig. 4.8 ]. A microcolon is an unused colon. The caliber of the postnatal colon depends on the amount of succus entericus which reaches it. If little or no succus reaches the colon, it will be of small caliber. If there is a proximal small bowel obstruction such as a duodenal atresia, the colon will be of normal caliber because the small bowel between the atresia and the colon is of sufficient length to produce a normal amount of succus. Thus with rare exceptions, a newborn with a microcolon must have a high-grade distal small-bowel obstruction: for all practical purposes, ileal atresia or meconium ileus. There are two exceptions to this rule: total colonic Hirschsprung disease (discussed later) and extreme prematurity. All very premature infants have microcolons related to underdevelopment. A contrast enema in these tiniest of babies rarely yields any useful information.
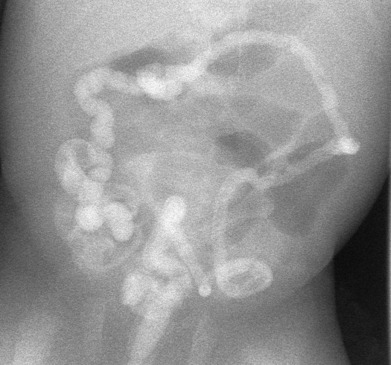
Hirschsprung Disease
Hirschsprung disease is the congenital absence of intestinal intramural ganglion cells resulting in failure of colonic relaxation and subsequent bowel obstruction. The aganglionic segment of bowel is continuous, extending proximally from the anus without “skip areas.”
Hirschsprung disease affects boys more than girls. It is usually not inherited, except in the relatively rare total colonic or total intestinal varieties. With the exception of trisomy 21, it is not usually associated with other anomalies.
Abdominal radiographs in newborns with Hirschsprung disease will show the typical pattern of low bowel obstruction. As in all newborns with presumed low obstruction, a contrast enema is the next step. The classic finding on the enema is a “transition zone” from nondilated, aganglionic distal bowel to relatively distended, normally innervated proximal bowel [ Fig. 4.9 ]. This transition zone will be best demonstrated on the lateral view, particularly in the phase of early filling. In most babies with Hirschsprung disease, the transition zone is gradual rather than abrupt, and thus may be relatively subtle.

The most common zone of transition on a contrast enema is the rectosigmoid colon; this is also the most common histopathologic zone of transition. The correlation between the radiologic and pathologic transition zones is quite good, but far from perfect. Intraoperative biopsy is necessary to document the absence of ganglion cells before proceeding to definitive treatment.
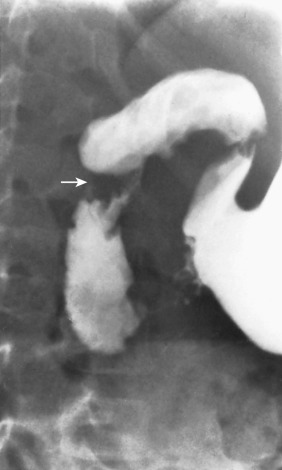
A helpful concept to keep in mind is the so-called rectosigmoid index. On a normal examination, the diameter of the rectum should be greater than the diameter of the sigmoid on all views. Thus the ratio of the diameter of the rectum to that of the sigmoid (the rectosigmoid index) should be greater than 1. In Hirschsprung disease, the index is reversed and the sigmoid is larger than the rectum [ Fig. 4.10 ]. Attention to this simple observation will prevent many missed diagnoses of Hirschsprung disease.

An additional fluoroscopic finding in Hirschsprung disease is a “sawtooth” appearance to the rectum related to irregular contractions in the aganglionic segment. Delayed evacuation of contrast material after the enema may also be noted. These findings are much less sensitive and specific than the transition zone. A small percentage of babies may present with clinical and imaging findings of enterocolitis, which may be severe.
In the rare patient with total colonic or total intestinal Hirschsprung disease, the findings on enema are variable and may include a true transition zone, in which the radiologic transition zone corresponds to the pathologic transition zone, a pseudotransition zone in which the radiologic and pathologic transition zones do not correspond, a short colon, or a microcolon.
Infrequently, the enema in a baby with Hirschsprung disease may be normal. Notably, this is the only condition that causes low bowel obstruction in which the enema may be normal. Thus any baby with a low obstruction and a normal enema should be presumed to have Hirschsprung disease until proven otherwise by biopsy.
Meconium Plug/Small Left Colon Syndrome
The cause of meconium plug syndrome, also called small left colon syndrome, is unknown. It is usually attributed to functional immaturity of the colon. There is an increased incidence in babies whose mothers have diabetes or have received magnesium sulfate for eclampsia. However, many affected babies have no identifiable risk factors. They present with low obstruction, but often have less severe symptoms than babies with other causes of bowel obstruction.
The contrast enema will demonstrate a normal-caliber rectum, a small-caliber sigmoid and descending colon, and an abrupt transition zone at the splenic flexure to a relatively distended transverse colon [ Fig. 4.11 ]. It cannot be stressed enough that the radiologist cannot make the diagnosis of meconium plug/small left colon syndrome in the absence of these findings. The rectum should be of normal caliber. If the rectum is as small as the left colon, Hirschsprung disease should be suspected [ Fig. 4.12 ]. The transition zone must be at the splenic flexure; a transition zone elsewhere suggests Hirschsprung disease. Meconium “plugs” may or may not be present. The amount of meconium in the colon of a newborn is a finding that is rarely of diagnostic value. It bears repeating that with a microcolon, the entire colon, not just the left colon, is small.


Many babies with functional immaturity will improve after the enema, and may in fact even pass the classic “meconium plug” on the fluoroscopy table. If the baby does not improve, a rectal biopsy should be performed to exclude Hirschsprung disease.
Colonic Atresia
Colonic atresia, like jejunoileal atresia, is caused by a prenatal vascular insult. Notably, newborns with colonic atresia may have additional areas of atresia.
Radiographs show a low bowel obstruction, often with a disproportionately dilated loop of bowel just proximal to the atretic segment [ Fig. 4.13 ]. When a radiograph demonstrates such a loop in a baby with obstruction at any level, an atresia should be suspected. Contrast enema shows a microcolon distal to an abrupt transition at the level of the atresia.

Meconium Ileus
Meconium ileus is a bowel obstruction seen almost exclusively in babies with CF. It is caused by inspissation of abnormally viscous meconium in the terminal ileum. The diagnosis is often made prenatally. The mother of a fetus found to have many dilated bowel loops on prenatal ultrasound may be tested for mutations causing CF. If the diagnosis is not made prenatally, meconium ileus remains in the differential diagnosis in a newborn with a low bowel obstruction. The finding of meconium ileus on enema should prompt an evaluation for CF.
Contrast enema shows a microcolon. As noted earlier, the finding of a microcolon essentially limits the differential diagnosis to either meconium ileus or ileal atresia. The presence of filling defects (pellets of abnormal meconium) impacted in a nondilated terminal ileum with progressive dilatation of the proximal small bowel is diagnostic of meconium ileus [ Fig. 4.14 ].

In 30% to 50% of newborns, meconium ileus may be “complicated.” In these babies, the massively dilated distal small bowel can twist upon itself, causing a segmental volvulus that may ultimately lead to ischemia and perforation. When perforation occurs, meconium spills into the peritoneal cavity and almost immediately calcifies. Radiographs show evidence of meconium peritonitis, with sheets of calcification around the liver, along the flanks, and in the deep pelvis [ Fig. 4.5 ]. Meconium peritonitis may notably occur in conditions other than meconium ileus when bowel perforation has occurred. Infrequently, a mass of dead bowel, fluid, and debris may form a meconium pseudocyst, the rim of which may calcify [ Fig. 4.6 ]. In some patients, a gasless abdomen may be seen. Unfortunately, radiographs in babies with complicated meconium ileus may in some cases be indistinguishable from those with uncomplicated meconium ileus.
Once a diagnosis of meconium ileus is made, the radiologist may be asked to perform a therapeutic contrast enema. Even though the technique of hydrostatic reduction with Gastrografin was introduced in 1969, there is still no consensus about the best way to do it. We have found that Gastrografin is more effective than other water-soluble agents. Notably, half-strength Gastrografin works just as well as full strength. Gastrografin is extremely hyperosmolar, and it presumably works by pulling water into the bowel and breaking up inspissated meconium. In our experience, the greatest predictor of failure of the procedure is a prior diagnostic enema with barium. This, as noted earlier, is one of the reasons for performing all diagnostic enemas in newborns with water-soluble contrast material. The goal of the radiologist should be to reflux contrast into the dilated distal small bowel. In babies who are stable and in whom the procedure appears to be working, several attempts can safely be made.
It cannot be stressed enough that reduction is a procedure that should be performed only by experienced radiologists in a center with experienced neonatologists. Even dilute Gastrografin is extremely hyperosmolar and may cause fluid shifts resulting in severe dehydration, electrolyte abnormalities, and even death. As indicated earlier, repeated attempts should be made only in babies who are clinically stable and in whom some therapeutic progress is being made. A lack of progress with reduction may imply that the baby has complicated meconium ileus. In babies with uncomplicated meconium ileus, the procedure is often successful and perforation is rare.
Ileal Atresia
Ileal atresia may be caused by either ischemic injury from an in utero vascular accident or mechanical obstruction, as may be seen with in utero volvulus related to meconium ileus. Radiographs show a low bowel obstruction, often with a disproportionately dilated loop of bowel. Contrast enema shows a microcolon. Unlike meconium ileus, the point of obstruction is not necessarily in the terminal ileum. If one is lucky enough to reflux beyond the ileocecal valve on contrast enema, the postatretic ileum will be of small caliber to the point of the obstruction [ Fig. 4.15 ].

Anorectal Malformation
Anorectal malformation, also commonly though inaccurately called imperforate anus, is likely the result of abnormal separation of genitourinary (GU) and hindgut structures in fetal life. This failure of separation results in an atresia of the rectum and a variable persistent communication between the GU and GI systems. This is analogous to the failure of separation of the respiratory and GI systems that results in EA and TEF (to be described).
Classification of the malformation is based on the position of the atretic rectum in relation to the levator sling. Atresias ending above the levator sling are classified as “high,” and those ending below it as “low.” The lesion may be further classified according to the anatomy of the termination. In most infants with high lesions, the atretic rectum ends in the GU tract. In boys, this is usually in the posterior urethra; in girls, it is typically in the vagina. In low lesions, there is usually a visible perineal orifice which may be stenotic or covered (“true” imperforate anus). There is not usually a communication with the GU tract.
The role of preoperative imaging is limited. Associated anomalies of the kidneys and spine are relatively common, and screening for these anomalies should be performed. After colostomy, the expected fistula to the urethra in boys may be delineated by a contrast study [ Fig. 4.16 ]. In girls, the communication is usually clinically obvious. Magnetic resonance imaging (MRI) can clearly define the musculature of the pelvic floor.

High Intestinal Obstruction
Obstruction of the Stomach
Congenital obstruction of the stomach, as may be seen in microgastria or pyloric atresia, is rare. Pyloric stenosis, although an extremely common cause of gastric outlet obstruction in infancy, almost never occurs in newborns younger than 1 week. A newborn with a dilated stomach is much more likely to have an obstruction of the duodenum than of the stomach.
Duodenal Obstruction
The duodenum is the most common site of intestinal atresia. Duodenal atresia is more common than duodenal stenosis or duodenal web. The cause of all three is notably the same: a failure of the solid fetal duodenum to recanalize. About 30% of infants with duodenal obstruction have trisomy 21.
Although duodenal atresia leads to complete duodenal obstruction, duodenal stenosis or web may be associated with partial or complete obstruction. An annular pancreas may be associated with duodenal atresia, stenosis, or web. Annular pancreas is due to a persistence of the embryonic ventral portion of pancreas, which encircles the second portion of the duodenum and contributes to obstruction.
Most infants with duodenal obstruction present with bilious vomiting within hours of birth because the site of obstruction in the setting of duodenal atresia, stenosis, or web is typically just distal to the ampulla of Vater. The diagnosis of duodenal atresia can usually be made from the abdominal radiograph alone. A “double bubble” is seen, reflecting air within a dilated stomach and proximal duodenum [ Fig. 4.17 ]. If distal gas is absent, duodenal atresia is the diagnosis. If distal gas is present, the diagnosis is usually duodenal stenosis or web, but importantly may also be malrotation with midgut volvulus (to be described). In all cases, surgery is usually the next step after radiographs, with the specific diagnosis made in the operating room.
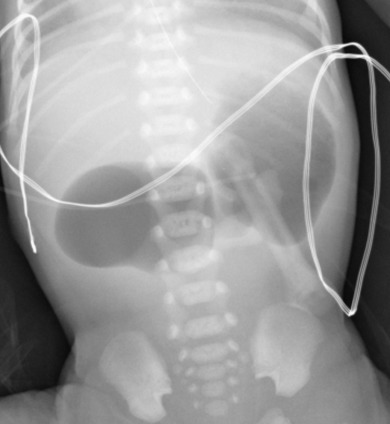
There is never a reason to administer contrast from above to a baby with a double bubble and no distal air on radiographs because a complete obstruction is clear. In this setting, air is an excellent contrast medium. In babies with partial obstruction, in contrast, the surgeon may request an upper GI series to differentiate duodenal stenosis or web from midgut volvulus. This distinction is key because duodenal stenosis and web do not require immediate surgery, whereas midgut volvulus is a surgical emergency. Many surgeons also request a contrast enema to exclude additional distal atresia(s).
On the upper GI of an infant with duodenal stenosis, contrast will outline a narrowing in the second portion of the duodenum [ Fig. 4.18 ]. If a duodenal web is present, it may appear as a thin, curvilinear filling defect in the lumen of the second portion of the duodenum [ Fig. 4.19 ]. Frequently, in babies with stenosis or web, the upper GI will simply demonstrate partial or complete duodenal obstruction. It is critically important to remember that obstruction caused by duodenal atresia, stenosis, or web occurs in the second portion of the duodenum. In contrast, obstruction caused by midgut volvulus occurs in the third portion.