Anatomy
8.1.1 Position and Divisions
All portions of the gastrointestinal tract—the esophagus, stomach, duodenum, jejunum, ileum, colon, and rectum—have the same general wall structure, which is adapted somewhat for specific regions. The wall layers that occur in all parts of the gastrointestinal tract are reviewed in ▶ Table 8.1.
Layers | Functions |
Mucosa | |
Epithelium | Superficial protection, hormone production, glandular cells (except for the esophagus) |
Lamina propria | Mobility, lymphatic tissue |
Muscularis mucosae | Fine control of mucosal folds |
Submucosa | Mobility, lymphatic tissue, Meissner plexus |
Muscularis propria | Mixing and transport actions: myenteric plexus (Auerbach’s plexus) between circular and longitudinal muscle layers |
Circular layer | |
Longitudinal layer | |
Adventitia | Connective tissue, incorporates the gastrointestinal tract into surrounding tissues |
Subserosa | Present only in intraperitoneal portions of the gastrointestinal tract |
Serosa | Present only in intraperitoneal portions of the gastrointestinal tract |
Lamina propria of serosa | |
Mesothelium | |
The esophagus is a hollow muscular tube, approximately 25 cm long, located in the mediastinum between the pharynx and stomach. It consists of three parts:
The cervical part.
The thoracic part.
The abdominal part.
The esophagus has three physiologic constrictions (▶ Fig. 8.1):
The upper esophageal constriction includes the upper esophageal sphincter behind the cricoid cartilage.
The middle esophageal constriction occurs where the esophagus is compressed by the aortic arch and tracheal bifurcation.
The lower esophageal constriction is located at the level of the diaphragm.

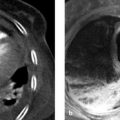
Fig. 8.1 Anatomy of the esophagus. (a) The trachea is anterior to the esophagus. The three physiologic constrictions are shown. (b) Structure of the esophageal wall in the contracted state (left) and relaxed state (right).
(Reproduced from Schünke M, Schulte E, Schumacher U. Prometheus. LernAtlas der Anatomie: Innere Organe. Illustrated by M. Voll /K. Wesker. 2nd ed. Stuttgart: Thieme; 2009.)
The mucosal lining of the esophagus (squamous epithelium) is marked by a pattern of longitudinal folds.
The stomach occupies an asymmetrical position in the left upper quadrant of the abdomen (▶ Fig. 8.2). It curves from the gastroesophageal junction (cardia) to the gastric outlet (pylorus). The upper part of the stomach is formed by the fundus, which is continuous with the main part, or body, of the stomach. The portion of the stomach proximal to the pylorus is called the antrum. The stomach has characteristic longitudinal rugal folds that start in the proximal fundus and run distally to the antrum. The rugal folds are prominent in the empty stomach and are effaced as the stomach becomes distended.

Fig. 8.2 Anatomy of the stomach. Schematic diagram.
The small intestine can be divided into three parts: the duodenum, jejunum, and ileum (▶ Fig. 8.3):
Duodenum: The duodenum is approximately 25 cm long, receives the common bile duct and pancreatic duct at the papilla of Vater, and merges with the jejunum at the ligament of Treitz. The C-shaped duodenum consists (from oral to aboral) of a superior part, a descending part, a horizontal part, and an ascending part. The inner surface of the duodenum is almost completely devoid of folds. The descending part has a short longitudinal fold, or plica, on which the pancreatic duct and common bile duct open at the papilla of Vater.
Jejunum: The jejunum is in the left upper quadrant of the abdomen and is approximately 2.0 to 2.5 meters long. Together with the ileum, its function is to absorb nutrients from ingested food. The mucosal lining of the jejunum is marked by transverse, crescent-shaped folds (Kerckring’s folds, valvulae conniventes).
Ileum: The ileum is located in the mid- and lower abdomen and is approximately 3 m long. Small mucosal folds are present only in its proximal portion. Instead, the ileum has nodular areas of lymphatic tissue (Peyer’s patches) on the side opposite the mesentery, which are grossly visible from the outside through the bowel wall. The ileum joins with the large intestine at the ileocecal valve.

Fig. 8.3 Anatomy of the small and large intestine. Schematic diagram.
The large intestine can be divided into three parts:
Cecum.
Colon.
Rectum (see ▶ Fig. 8.3).
The colon, in turn, consists of several segments:
Ascending colon.
Transverse colon.
Descending colon.
Sigmoid colon.
Note
The gastrointestinal tract has both intraperitoneal and retroperitoneal portions. The following portions are retroperitoneal:
Duodenum (except for the ascending part.)
Ascending colon.
Descending colon.
Rectum.
The total length of the colon ranges from 100 to 170 cm. The wall structure of the colon is markedly different from that of the small intestine and rectum. It is characterized by three separate, longitudinal bands of smooth muscle fibers in the colon wall (teniae coli). Another feature is an alternation between sacculations of the colon wall (haustra) and crescent-shaped constrictions (semilunar folds). The rectum is characterized by several transverse mucosal folds. The middle and most prominent transverse fold, called Kohlrausch’s fold, is situated approximately 5 to 8 cm proximal to the anus and marks the start of the retroperitoneal part of the rectum. Viewed in longitudinal section, the muscular layer of the rectum is not bundled into teniae as in the colon but forms a uniform longitudinal coat. Viewed in cross section, the muscular layer of the rectum is thickened above the anus to form a constricting ring, the internal anal sphincter.
8.1.2 Imaging Landmarks
Radiographic landmarks ▶ Fig. 8.4 shows the radiographic landmarks for the esophagus, and ▶ Fig. 8.5 shows the landmarks for the gastrointestinal tract. In most cases, delineation of the gastrointestinal tract requires the administration of contrast material such as certain barium compounds or iodinated contrast media.

Fig. 8.4 Anatomy of the esophagus. Oral contrast examination. (a) AP projection. (b) Lateral projection.

Fig. 8.5 Anatomy of the stomach and large intestine. (a) Anatomy of the stomach in the left upper abdomen after oral administration of contrast medium. (b) Anatomy of the ascending, transverse and descending colon after rectal administration of contrast medium.
Sonographic landmarks Ultrasound displays the typical stratified wall structure of the gastrointestinal tract, consisting of the hypoechoic mucosa, hyperechoic submucosa, and hypoechoic muscularis propria (▶ Fig. 8.6).

Fig. 8.6 Gastrointestinal wall. Ultrasound appearance. The mucosa is hypoechoic, the submucosa is hyperechoic, and the muscularis propria is hypoechoic.
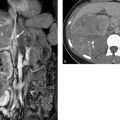
Landmarks in CT and MRI As in conventional radiography, gastrointestinal structures are demonstrated more clearly by CT and MRI when orally and/or rectally administered contrast media are used. Without contrast medium, portions of the stomach and bowel are often in a collapsed state and are poorly delineated from neighboring structures. Moreover, diagnostic evaluation of the gastrointestinal wall is significantly more difficult without contrast material. The esophagus descends between the trachea and the aorta in the posterior superior mediastinum. Its distal portion borders on the left atrium. The anterior stomach wall is in contact with the liver on the left side and the spleen on the right side. The stomach relates posteriorly to the pancreas and inferiorly to the transverse colon (▶ Fig. 8.7). Imaging landmarks for the small and large intestine are illustrated in ▶ Fig. 8.8 and ▶ Fig. 8.9.

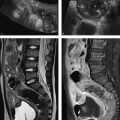
Fig. 8.7 Stomach and adjacent organs. Axial CT scans. (a) Solid arrow, stomach; dashed arrow. colon; cross, spleen; asterisk, liver. (b) Solid arrow, stomach; dashed arrow, pancreas: cross, spleen; asterisk, liver.

Fig. 8.8 Sectional imaging landmarks for the small intestine. Coronal MR images. (a) The jejunum (arrow). (b) The ileum (arrow). The transverse colon (TC) and urinary bladder (B) are also shown.

Fig. 8.9 Sectional imaging of the colon. Limbs of the colon displayed by T2W MRI. The asterisk indicates small bowel loops and mesenteric structures enclosed by the colon. AC, ascending colon; DC, descending colon; TC, transverse colon.
8.2 Imaging
8.2.1 Radiography
Fluoroscopic examination with oral contrast medium still has a major role in imaging studies of the esophagus.
Caution
The use of barium contrast medium is contraindicated in patients with a suspected esophageal perforation. Also, a water-soluble iodinated iso-osmotic contrast medium should be used if there is a risk of aspiration (test with a sip of water).
Disease of the gastrointestinal tract have a diverse etiology that mainly includes inflammations, infections, and tumors. The indications for a plain abdominal radiograph, which should be taken in the supine or left lateral decubitus (LLD) position, are limited to a few conditions such as bowel obstruction (▶ Fig. 8.10) or suspected free air originating from a perforation or dehiscent suture line (▶ Fig. 8.11). Abdominal radiographs may also be obtained to evaluate the transit of oral contrast medium and check for lesions that could delay gastrointestinal transit, such as tumors or bowel adhesions.

Fig. 8.10 Small-bowel obstruction. Conventional radiograph in left lateral decubitus shows moderately distended small-bowel loops with multiple air–fluid levels (arrows) consistent with a small-bowel obstruction.

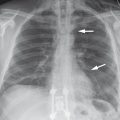
Fig. 8.11 Free air. Free air is visible along the dome of the liver (arrow) in a patient with a perforated bowel.
8.2.2 Computed tomography and Magnetic Resonance Imaging
Computed tomography (CT) is the imaging modality of choice for acute clinical diagnosis and for staging. Magnetic resonance imaging (MRI) is preferred for nonacute indications (e.g., follow-ups).
Note
With few exceptions, sectional imaging modalities like CT and MRI have replaced conventional radiographs in the evaluation of gastrointestinal diseases.
8.2.3 Contrast Media
The use of oral or rectal contrast media often provides great diagnostic benefit:
They help define the wall of the gastrointestinal tract and delineate it from the lumen.
They help in distinguishing bowel from mesenteric or retroperitoneal structures.
Conventional imaging studies employ barium-containing or iodinated media as positive contrast agents. Barium-based media should not be used in patients with a suspected perforation of the gastrointestinal tract, as they could incite a severe peritonitis. Recent studies have shown that negative contrast media such as mannitol or mineral water (1 liter ingested over a 30-minute period) are better than positive media for evaluating the intestinal wall on CT. Gastrografin, a water-soluble radiopaque contrast medium, should be used in patients with a suspected perforation because contrast extravasation into the free abdominal cavity would be indistinguishable from ascites if a negative medium was used. Water-based contrast media (e.g., mannitol solutions) can also be used in MRI.
8.3 Diseases of the Esophagus
8.3.1 Achalasia
Brief definition Achalasia is a functional disorder of the smooth muscle of the lower esophageal sphincter secondary to degenerative changes in the myenteric plexus. It results in a failure of lower esophageal sphincter relaxation during swallowing. The prevalence of achalasia is approximately 10:100,000 population per year, with a peak age incidence in the third and fourth decades.
Note
Achalasia progresses in three stages:
Hypermotile stage.
Hypomotile stage.
Amotile stage.
During the first stage, the proximal part of the esophagus increases its motility in an effort to surmount the increased distal resistance. Further progression is marked by increasing damage to the esophageal muscles and diminished contractions.
Imaging signs Fluoroscopic imaging (▶ Fig. 8.12) shows dilatation of the thoracic esophagus with funnel-shaped or “bird-beak” narrowing of the distal esophagus. The passage of contrast medium through the gastroesophageal junction is greatly delayed.

Fig. 8.12 Achalasia. Radiograph after oral administration of contrast medium. The distal esophagus is tapered with a “champagne glass” appearance.
Clinical features Patients typically present with dysphagia, which is usually associated with regurgitation (and possible aspiration) and retrosternal pain. Initial treatment consists of pharmacologic agents such as calcium-channel blockers to reduce the muscle tone of the lower esophageal sphincter. Other treatment options are balloon dilation (which may be repeated as required), endoscopic botulinum injection, and surgery.
Differential diagnosis It is important to exclude a secondary cause of lower esophageal narrowing, such as a malignant tumor. In this case, however, a mass lesion would be found at imaging, whereas primary achalasia would not be associated with a detectable mass. The differential diagnosis also includes scleroderma, which is distinguished by generalized dilatation and dysmotility of the esophagus.
Key points Before achalasia is diagnosed, it is important to exclude a different cause of esophageal obstruction, particularly a malignant tumor.
8.3.2 Esophageal Diverticula
Brief definition Esophageal diverticula are outpouchings that involve one or more layers of the esophageal wall. Two main types are distinguished:
Pulsion diverticula: These are pseudodiverticula that result from increased intraluminal pressure and involve the herniation of mucosa and submucosa through the muscularis propria.
Traction diverticula: These are true diverticula that involve the herniation of all wall layers. They usually result from pulling forces on the outer aspect of the esophagus.
Pulsion diverticula are far more common than traction diverticula. Esophageal diverticula are classified by their location (▶ Fig. 8.13):
Zenker’s diverticulum: a pulsion diverticulum caused by weakness of the cervical esophagus just above the cricopharyngeus muscle.
Parabronchial diverticulum: a traction diverticulum usually caused by inflammation of lymph nodes with subsequent scarring and adhesion of matted nodes to the esophagus.
Epiphrenic diverticulum: a pulsion diverticulum of the distal esophagus that results from a swallowing disorder in which the intraluminal pressure is raised before the food bolus enters the stomach.

Fig. 8.13 Location and frequency distribution of esophageal diverticula. Schematic diagram.
(Reproduced from Schünke M, Schulte E, Schumacher U. Prometheus. LernAtlas der Anatomie: Innere Organe. Illustrated by M. Voll /K. Wesker. 2nd ed. Stuttgart: Thieme; 2009.)
Imaging signs Most esophageal diverticula are clearly detectable as outpouchings in the oral contrast study. The examination should always be performed in two stages. After the contrast medium bolus has been swallowed and has passed through the esophagus, contrast medium will tend to pool in the diverticulum, and the retained contrast medium will remain visible for some time (▶ Fig. 8.14).

Fig. 8.14 Parabronchial esophageal diverticulum. Contrast medium has been retained in a diverticulum (arrow) following oral contrast examination.
Clinical features Esophageal diverticula are usually asymptomatic, but some patients complain of dysphagia and a foreign-body sensation. Foul breath odor may result from retained food residues in the diverticulum. Complications such as fistulation or rupture are rare. Very large, symptomatic diverticula should be surgically removed.
Differential diagnosis Diverticula have very typical imaging features, so generally there is no need to consider other possible entities. Hiatal hernias can sometimes mimic diverticula on sectional images, but differentiation is easily accomplished by oral contrast examination.
Key points Esophageal diverticula are usually asymptomatic. Oral contrast examination is diagnostic.
8.3.3 Esophageal Varices
Brief definition Esophageal varices are enlarged submucosal vessels in the esophageal wall that develop in response to portal hypertension. The portal hypertension, usually secondary to hepatic cirrhosis, stimulates the development of collateral blood flow (through the short gastric veins to the esophageal veins), causing enlargement of the esophageal vessels and the development of a portocaval shunt.
Imaging signs Contrast-enhanced CT or MRI can clearly demonstrate the convoluted varices in or on the esophageal wall (▶ Fig. 8.15). On fluoroscopic examination with oral contrast medium, esophageal varices appear as tortuous, longitudinal filling defects in the esophageal lumen (▶ Fig. 8.16).

Fig. 8.15 Varices in and on the esophageal wall. Axial CT scan shows marked enhancement and tortuosity of the varices (arrow).

Fig. 8.16 Esophageal varices. Esophageal varices appear as longitudinal filling defects on oral contrast examination.
Clinical features Esophageal varices may be clinically silent initially. Very large varices may lead to narrowing or obstruction of the esophageal lumen. It is common to find associated signs of portal hypertension such as splenomegaly or ascites.
Note
Rupture and bleeding of the thin-walled esophageal varices is a life-threatening complication that requires prompt endoscopic sclerotherapy.
If sclerotherapy cannot be undertaken right away, a Sengstaken–Blakemore tube with an inflatable balloon can be passed down the esophagus as a temporizing measure. The mortality rate of acute variceal hemorrhage is 30%. In patients with recurrent bleeding, a transjugular intrahepatic portosystemic shunt can be placed to lower the portal venous pressure and reduce the bleeding risk.
Differential diagnosis Only a few entities need to be considered in the differential diagnosis. Hiatal hernias may resemble esophageal varices on sectional images but will generally contain air–fluid levels. Other masses such as esophageal carcinoma or mediastinal lymph nodes do not display the typical longitudinal orientation or tortuosity of esophageal varices. Another helpful differentiating criterion is given by the fact that esophageal varices almost always occur in a setting of hepatic cirrhosis.
Key points Esophageal varices have a characteristic imaging appearance with a very limited differential diagnosis.
8.3.4 Esophageal Atresia and Tracheoesophageal Fistula
Brief definition Esophageal atresia is a congenital interruption of the esophagus, usually with an associated tracheoesophageal fistula. Esophageal atresia has a reported prevalence of 1 in 3,000 to 4,000 neonates. The most common form, accounting for 90% of cases, is Vogt type IIIb (▶ Table 8.2, ▶ Fig. 8.17). Esophageal atresia is usually combined with other anomalies in the VACTERL association:
V: vertebral anomalies.
A: anal atresia.
C: cardiac anomalies.
TE: tracheoesophageal fistula.
R: renal anomalies.
L: limb deformities.

Fig. 8.17 Vogt classification of esophageal atresia.
Table 8.2 Vogt classification of esophageal atresia
Type
Description
I
Aplasia
II
Atresia without a fistula
IIIa
Atresia with a proximal fistula
IIIb
Atresia with a distal fistula
IIIc
Combination of types IIIa and IIIb
IV
Tracheoesophageal fistula without atresia (H-type fistula)
Imaging signs Findings on plain chest and abdominal radiographs are variable, depending on the type of atresia. Types I, II, and IIIa are associated with an air-free abdomen, while types IIIb and IIIc are characterized by air in the upper abdomen. It is common to find pulmonary changes due to aspiration. Oral administration of contrast medium demonstrates pooling of contrast medium within a blind pouch.
Clinical features Clinical manifestations include coughing and drooling in neonates, with a deterioration of clinical status. Suspicion of esophageal atresia can be confirmed by introducing a gastric tube. If atresia is present, the tube cannot be passed into the stomach, and gastric juices cannot be aspirated (▶ Fig. 8.18). Treatment is surgical and consists of an end-to-end anastomosis of the esophagus or reconstruction by an esophagoplasty. Surgery includes the repair of tracheoesophageal fistulas.

Fig. 8.18 Type IIb esophageal atresia. Conventional radiograph of a newborn shows a stomach tube doubled back in an esophageal blind pouch (arrows). The air-filled stomach suggests the presence of a tracheoesophageal fistula.
Differential diagnosis The differential diagnosis includes high-grade esophageal strictures or stenoses, but these lesions are rare in neonates.
Key points Radiological findings depend on the type of atresia. It is also important to look for possible associated anomalies.
8.3.5 Hiatal Hernia
Brief definition A hiatal hernia occurs when portions of the stomach herniate into the chest through the esophageal hiatus of the diaphragm. Two main types are distinguished:
Axial sliding hernia: In this type the gastric cardia slides through the esophageal hiatus into the mediastinum (▶ Fig. 8.19). The gastroesophageal junction is intrathoracic.
Paraesophageal hernia: Portions of the stomach herniate through the esophageal hiatus alongside the esophagus. The gastroesophageal junction is intra-abdominal. The extreme form is an “upside-down stomach” (▶ Fig. 8.20).

Fig. 8.19 Para-axial sliding hernia. CT shows displacement of the gastric cardia into the chest (arrow).

Fig. 8.20 Paraesophageal hernia with an “upside-down stomach.” The esophagus (open arrowhead) is visible in the same section as intrathoracic portions of the herniated stomach (arrows).
Imaging signs Plain radiography and sectional imaging demonstrate an intrathoracic or retrocardiac mass that is partially air-filled and is closely related to the diaphragm (▶ Fig. 8.21).

Fig. 8.21 Hiatal hernia. Lateral chest radiograph demonstrates the hiatal hernia as a partially air-filled retrocardiac mass (arrow). Previous aortic valve replacement is noted as an incidental finding.
Clinical features Hiatal hernia is usually asymptomatic, but loss of the functional esophageal sphincter may lead to increased gastroesophageal reflux. This can produce symptoms of reflux disease such as heartburn, regurgitation, and pain. Barrett’s esophagus will develop in approximately 10% of patients with reflux disease. This condition results from chronic inflammation of the esophagus due to acid reflux and is characterized by a metaplasia that transforms the nonkeratinized stratified squamous epithelium of the esophagus into a single layer of columnar epithelium. Barrett’s esophagus is considered a precancerous condition with a 40-fold increase in cancer risk relative to the normal population. In addition, large hernias may compromise pulmonary ventilation. Axial sliding hernia with concomitant reflux disease can be treated by fundoplication. Paraesophageal hernias should be surgically repaired because of the risk of incarceration or volvulus.
Differential diagnosis The differential diagnosis includes other masses of the posterior mediastinum such as neurogenic tumors or pericardial cysts, but these lesions do not contain air. Bronchogenic cysts may be difficult to distinguish from hiatal hernia because they may also contain air if they communicate with the bronchial tree or esophagus. Oral contrast examination can reliably differentiate these conditions, however.
Key points Hiatal hernias are a common finding in older patients and are usually asymptomatic.
8.3.6 Esophageal Carcinoma
Brief definition Esophageal carcinoma is a malignant tumor of the esophagus. The two main types are squamous cell carcinoma (70–80% of cases) and adenocarcinoma (20–30%). The risk of developing squamous cell carcinoma is increased in smokers, heavy drinkers, and patients with achalasia. Adenocarcinoma often develops in patients with a Barrett’s esophagus. The peak age of incidence is in the sixth and seventh decades.
Imaging signs The contrast swallow study shows irregular narrowing of the esophageal lumen, sometimes with associated ulceration of the esophageal mucosa (▶ Fig. 8.22). Sectional imaging can define the depth of mediastinal invasion and provide information on lymph node status and possible distant metastasis (▶ Fig. 8.23).

Fig. 8.22 Esophageal carcinoma. Oral contrast examination in a patient with esophageal carcinoma shows dilatation of the proximal esophagus and irregular narrowing of the distal esophagus caused by the tumor (arrows).

Fig. 8.23 Large esophageal carcinoma. CT can accurately define the extent of tumor involvement (arrow).
Clinical features Esophageal carcinoma is associated with dysphagia and weight loss. The TNM classification of esophageal carcinoma is shown in ▶ Table 8.3. Treatment options (surgery, chemoradiotherapy) are determined by the UICC (Union for International Cancer Control) stage of the disease (▶ Table 8.4).
Designation | Description |
Primary tumor | |
Tis | Carcinoma in situ (high-grade dysplasia) |
T1 | Tumor invades the lamina propria or submucosa |
T2 | Tumor invades the muscularis propria |
T3 | Tumor invades the adventitia |
T4 | Tumor invades adjacent structures |
Lymph nodes | |
N0 | No regional lymph node metastasis |
N1 | Regional lymph node metastasis |
Distant metastasis | |
M0 | No distant metastasis |
M1 | Distant metastasis |
Stage | Groupings |
I | T1 N0 M0 |
IIa | T2–3 N0 M0 |
IIb | T1–2 N1 M0 |
III | T3 N1 M0 or T4 N0–1 M0 |
IV | T1–4 N0–1 M1 |
Differential diagnosis The differential diagnosis includes esophageal strictures due to other causes (e.g., peptic strictures) and achalasia, which is distinguished by a smooth, less irregular mucosal lining. Other tumors of the esophagus, such as leiomyomas, are rare.
Key points The primary tumor can be clearly visualized in a contrast swallow study. CT is used mainly for the staging of disease.
8.4 Diseases of the Gastroduodenum
Because many pathologic changes in the stomach and duodenum occur at the mucosal level, imaging studies are often less rewarding than endoscopy. Nevertheless, imaging plays a crucial role in emergency situations (acute abdomen, suspected perforation) and the staging of malignant disease.
8.4.1 Hypertrophic Pyloric Stenosis
Brief definition This condition is a narrowing of the gastric outlet due to thickening of the circular muscle layer and swelling of the mucosa. The disorder occurs predominantly in male infants 3 to 7 weeks old. Its prevalence in the western population is estimated at 1 in 800 infants. Pyloric stenosis is virtually unknown in the Asian and African populations. The etiology of hypertrophic pyloric stenosis is not yet fully understood.
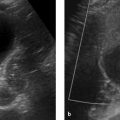
Imaging signs Ultrasound demonstrates hyperperistalsis of the strongly fluid-distended stomach. The pyloric canal is elongated (>16 mm) with thickened musculature (>4 mm) and a small lumen (▶ Fig. 8.24). Thoracic and abdominal radiographs show a large stomach and an under-aerated small intestine. Pulmonary changes due to aspiration are a common finding.

Fig. 8.24 Pyloric stenosis. Ultrasound appearance. (a) Infant with a normal pylorus. (b) Infant with pyloric stenosis. The scan shows marked thickening of the muscular layer at the gastric outlet (arrows). (c) Same infant as in [b]. This scan also shows a thickened muscular layer at the gastric outlet (arrows) and a distended, fluid-filled gastric bubble (asterisk).
Clinical features Pyloric stenosis impairs the passage of gastric contents, and infants typically present with projectile vomiting after feeding. The loss of gastric acid leads to metabolic hyperchloremic alkalosis. Physical examination may reveal a palpable pyloric mass (“palpable olive”).
Differential diagnosis Narrowing of the gastric outlet may also result from a tumor or from scarring due to ulcerative disease. However, these conditions would affect a different age group than hypertrophic pyloric stenosis, and an underlying disease (tumor, inflammation) would also be present.
Key points Hypertrophic pyloric stenosis presents typical ultrasound imaging features that are usually diagnostic when correlated with clinical findings.
8.4.2 Diverticula
Brief definition A gastric or duodenal diverticulum is an outpouching of the wall layers in the upper gastrointestinal tract. Diverticula of the stomach are most commonly located in the gastric cardia (▶ Fig. 8.25). Duodenal diverticula are often located in the descending part of the duodenum (▶ Fig. 8.26).

Fig. 8.25 Diverticulum of the gastric cardia. CT appearance. (a) Water-filled diverticulum (arrow). (b) Diverticulum filled with oral contrast medium (arrow).

Fig. 8.26 Large duodenal diverticulum. The arrows indicate the diverticulum. (a) Axial CT. (b) Coronal CT.
Imaging signs Air–fluid levels are commonly found in diverticula. When barium or another oral contrast medium is administered, fluoroscopy or conventional radiography will often show contrast medium pooling in the diverticulum. On sectional imaging by CT or MRI, a diverticulum will show only peripheral enhancement after IV injection of contrast medium, while the central (air- or fluid-filled) areas do not enhance. This effect is more clearly appreciated with a negative contrast medium such as mannitol solution than with a positive contrast medium like barium or an iodinated medium.
Clinical features Most diverticula are asymptomatic and do not require treatment. However, the infection of a diverticulum (diverticulitis) may cause significant symptoms such as pain. Treatment options range from antibiotic therapy to surgery, depending on the severity of symptoms.
Differential diagnosis Diverticula at certain sites can mimic masses of the stomach, duodenum, or other organs. These include tumors of the adrenal gland, spleen, or pancreas. Abscesses may also resemble diverticula. The two imaging hallmarks—the pooling of swallowed contrast medium in the diverticular lumen and peripheral enhancement after IV injection of contrast medium—are important imaging clues that distinguish diverticula from other lesions of the gastric or duodenal wall.
Key points Diverticula are rare, benign lesions of the upper gastrointestinal tract. The use of oral or IV contrast media is helpful in differentiating diverticula from tumors and abscesses.
8.4.3 Peptic Ulcer Disease
Brief definition of peptic ulcer disease (PUD) A peptic ulcer is a defect in the gastric or duodenal wall that penetrates past the level of the mucosa and submucosa to involve deeper wall layers. The pathogenesis of ulcer disease involves an imbalance between protective and aggressive factors (e.g., gastritis, Helicobacter pylori infection, use of NSAIDs, stressful situations such as intensive care). Peptic ulcers are most commonly located in the lesser curvature of the stomach or the duodenal bulb.
Imaging signs Fluoroscopy with contrast medium usually shows a well-circumscribed area of contrast medium pooling in the ulcer niche. With a perforated ulcer, free air can be detected by a plain abdominal radiograph in LLD or by CT (▶ Fig. 8.27).

Fig. 8.27 Perforated ulcer. Free intra-abdominal air ([a], [b], arrows) is detected on axial CT in a patient with a perforated ulcer. A significant volume of ascites ([a], crosses) is also present. (a) Soft tissue window. (b) Lung window.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree