Key Facts
- •
Gaucher’s disease is an autosomal recessive condition.
- •
Deficiency of beta glucosidase leads to accumulation of glucosylceramide in lysosomes of monocyte/macrophage lineage.
- •
Hepatosplenomegaly and replacement of bone marrow are typically present; central nervous system changes occur in some cases.
- •
Musculoskeletal findings include marrow replacement, osteopenia, focal lytic lesions (“Gaucheromas”), acute focal bone disease (osteonecrosis, osteomyelitis or pseudoosteomyelitis, fracture), and Erlenmeyer flask deformity of the femurs.
- •
Magnetic resonance imaging (MRI) shows homogeneous or patchy low-signal intensity marrow on both T1-weighted and T2-weighted imaging sequences.
- •
Marrow “reconversion” occurs with replacement of the proximal marrow and formation of red marrow further peripherally.
- •
MRI is the most effective method of identifying acute infarction.
- •
Bone scans are more useful than MRI for distinguishing acute infarcts from acute osteomyelitis, as photopenia is present in the first 3 days after infarction.
Gaucher’s disease (GD) is due to a genetic deficiency of the enzyme beta glucosidase.
GD is an autosomal recessive condition characterized by a deficiency of beta glucosidase.
This functional deficit results in accumulation of a cell membrane metabolite, glucosylceramide, in lysosomes of cells of the monocyte/macrophage lineage. The condition is named for Philippe Gaucher, a French dermatologist who first described the clinical syndrome. GD is the most common of the lysosomal storage diseases and results in a high frequency of skeletal symptoms.
More than 300 mutations that may result in clinical GD have been identified; seven alleles account for the majority of nucleotide substitutions, four of which are responsible for approximately 90% of cases. Inheritance is autosomal recessive. The condition is widely distributed in the population but is particularly prevalent among the Ashkenazi Jews, in whom 1 in 14 may be carriers.
Diagnosis of GD is typically made by enzyme assay, DNA analysis, bone marrow biopsy, spleen or liver biopsy, or some combination of these four methods. Of these, assay for glucocerebrosidase activity of peripheral blood leukocytes is considered to be the most efficient and reliable means of diagnosis.
Diagnosis is confirmed by assay for glucocerebrosidase in leuckocytes.
However, GD was recognized prior to the introduction of enzyme assay by its clinical features, which are the result of accumulation of glucosylceramide in cells of the reticuloendothelial system. Apoptosis is inhibited in these cells, and their relative immortality leads to accumulation of characteristic Gaucher cells, causing hepatosplenomegaly and replacement of normal marrow elements. The severity of disease is widely variable. In general, the younger the age at presentation, the more severe the condition.
In general, the younger the age at presentation, the more severe the condition.
In rare instances, severely affected individuals have been identified as early as the second trimester of pregnancy by the presence of polyhydramnios, hydrops fetalis with bilateral hydrothorax, hepatosplenomegaly, arthrogryposis, absent fetal movements, and thickened skin.
The presence and degree of central nervous system (CNS) involvement has been used to delineate three more-or-less distinct clinical forms of the disease. The most prevalent form of GD (type 1) lacks primary involvement of the CNS ( Box 27-1 ).
TYPE 1 (ADULT TYPE)
No CNS involvement
TYPE 2
Infantile onset
Severe CNS disease
Death in childhood
TYPE 3
Adolescent or early adult onset
Milder CNS involvement
Traditionally, this has been referred to as the “adult type”; however, 66% of individuals with Type 1 GD manifest symptoms in childhood. The most severe variants of type 1 GD may cause death at an early age due to visceral and hematologic manifestations, although the more common forms are compatible with a normal life expectancy.
Type 2 is characterized by onset in infancy with severe CNS involvement and death in early childhood. Type 3 demonstrates milder CNS involvement with onset in adolescence or early adulthood and a more indolent course.
OSTEOARTICULAR IMAGING FEATURES
Skeletal complications appear slowly over time, and therefore skeletal manifestations are less characteristic of the more fulminant forms of the disease. However, when skeletal disease is present in the more severe clinical forms, the same spectrum of radiographic findings is seen as in type 1 disease. Vertebral compression fractures and osteonecrosis of the long bones occur frequently.
For many patients with type 1 disease, the skeletal manifestations are probably the most disabling aspect. Although some affected individuals can be asymptomatic with neither radiographic, scintigraphic, nor histologic evidence of bone involvement, this is the exception. Findings from the International Collaborative Gaucher Group Registry, an international database of more than 2600 patients, show that nearly all patients with GD have radiologic evidence of skeletal involvement, and the majority has a history of serious skeletal complications.
The skeleton is not affected uniformly but rather tends to have focal or multifocal manifestations, and sometimes much of the skeleton is preserved. These observations suggest that bone is affected because of collections of Gaucher cells scattered throughout its substance. It is possible that local effects may be the result of a toxic process around these foci. Alternatively, the storage of glucocerebroside in tissue macrophages may disturb the generation of competent osteoclasts and thus result in a failure to maintain a healthy skeleton.
Patients commonly experience nonspecific bone pain, and some suffer from intermittent episodes of severe pain (bone crises) similar to those seen in sickle cell disease. Up to 20% have impaired mobility.
Radiographically demonstrable involvement results from five basic processes:
- 1
Marrow replacement
- 2
Generalized osteopenia
- 3
Skeletal resorption due to adjacent heavily involved marrow leading to focal lytic lesions
- 4
Acute focal bone disease including:
- •
Osteonecrosis, especially collapse of the femoral head
- •
Osteomyelitis and “pseudoosteomyelitis”
- •
Fractures
- •
- 5
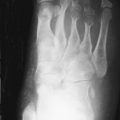
The Erlenmeyer flask deformity, a characteristic (but not universally present) modeling abnormality of the distal femur
The Erlenmeyer flask deformity is the characteristic modeling abnormality of the distal femurs in patients with GD.
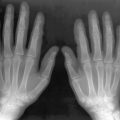
Bone Marrow Replacement
Marrow replacement is the most universally present skeletal manifestation of GD. Normal bone marrow becomes infiltrated by cellular elements containing foam cells (macrophages packed with glucocerebroside). This feature is impossible to recognize on radiographs and is extremely subtle on CT scans but quite obvious on MRI. Marrow affected by GD characteristically demonstrates either homogeneous or patchy low-signal intensity on both T1-weighted and T2-weighted imaging sequences. These signal alterations presumably reflect the combined effects of replacement of marrow fat and the highly structured microtubular arrays in which the glucocerebroside accumulates.
During normal development, the maximal extent of red marrow occurs at the ninth month in utero. Subsequently the red marrow is progressively replaced by yellow or fatty marrow, beginning in the distal parts of the appendicular skeleton and proceeding proximally corresponding with increases in the marrow signal on T1-weighted images.
By age 6, adult levels of fat content (40% to 45%) are reached in the posterior ilium. By age 10, marrow signal intensity in most pelvic sites is similar to that of adults, although fatty replacement remains ongoing throughout life. Most normal epiphyseal centers have fatty marrow throughout life, speculatively because the physiologic requirement for red marrow is declining by the time in childhood that ossification of the secondary centers occurs. Therefore the normal pattern of marrow conversion is centripetal, except for the fact that the secondary centers do not participate.
In GD, the process of fatty marrow conversion is reversed, with accumulation of GD-affected tissue beginning in the axial skeleton and proceeding into the proximal and then the distal long bones. The epiphyses are relatively spared, although with advanced disease they too are affected ( Figure 27-1 ).

Gaucher tissue is deposited first in areas of red marrow, which leads to reconversion of peripheral yellow marrow to hematopoietic marrow.
This pattern of disease progression is thought to reflect the availability of the macrophages that serve as reservoirs for the glucocerebroside accumulation. Such macrophages are primarily found in red marrow, and therefore areas of residual red marrow tend to be affected first. Replacement of the proximal marrow induces hematopoiesis with formation red marrow further peripherally, and Gaucher accumulation follows. This reverse process is known as reconversion and may also be seen in other marrow-replacing disorders.
Several approaches have been devised to quantify the extent of marrow replacement. Simple scales based upon the known pattern of disease progression and visual determination of the presence of disease have been developed by Rosenthal and Hermann ( Figure 27-2 ). These scales are valuable because the extent of marrow replacement is related to the other indices of disease severity, indicates the probability of skeletal symptoms, and may help to guide the need for therapy.

Because the vertebral marrow appears darker than expected on T1-weighted images due to absence of fat, one “semi-quantitative” approach to determine whether the marrow is affected by GD is to compare vertebral body signal intensity to disk brightness. If the normalized vertebra to disk ratio (NVDR) is taken to be 1.0 in controls (95% confidence limits 0.70 to 1.30), for untreated patients with GD the ratio is below 0.7.
Gaucher vertebrae are darker than normal on T1-weighted images due to the absence of fat.
It is also possible to calculate a truly quantitative value for the extent of marrow disease, which is inversely related to the remaining volume percent of fat (fat fraction). Fat fraction is generally calculated using the technique of quantitative chemical shift imaging. It is decreased severalfold in GD patients compared with normal individuals.
A correlation has been demonstrated between fat fraction of axial bone marrow as calculated by Dixon quantitative chemical shift imaging (Dixon QCSI) and bone complications. There is an 85% increase in risk of bone complication for every decrement of 0.1 in the fat fraction. Furthermore, fat fractions are significantly lower in GD patients who have undergone splenectomy than in those who have not. The latter observation supports the clinical impression that removal of the splenic “reservoir” may worsen the skeletal disease. Other studies have also demonstrated a correlation between fat fraction and clinical disease severity, although not always with bone complications.
Splenectomy may worsen bone disease.
Radioisotope scans have also been used to evaluate the marrow changes of GD. Whole body scans performed using inhaled xenon-133 have been shown to correlate with the extent of skeletal disease. The extent of marrow involvement as determined by Tc-99m sulfur colloid correlated well with the clinical and radiologic changes of the skeleton, but a normal pattern was found in the early stages of the disease. Tc-99m sestamibi (MIBI) has also been utilized for direct visualization of glycolipid deposits in the bone marrow. Although MIBI scanning is a sensitive technique for detecting bone marrow deposits, there is no clear correlation between the observed uptake and clinical disease. Some studies suggest that MIBI scans are inadequate for early identification of patients at high risk for skeletal complications or for the follow-up of patients treated with enzyme replacement. Others have found that a semiquantitative scintigraphic score was highly correlated with an overall clinical severity score index (SSI) and with various parameters contributing to the SSI, either positively or negatively. Scintigraphic score is most highly correlated with measurements of serum chitotriosidase, an overall biochemical marker of disease severity. Enzyme replacement therapy (ERT)-naive patients showed high correlation of the scintigraphic score with the clinical SSI, with a radiographically based score, and with serum chitotriosidase. In the ERT-treated patients, the scintigraphic score was correlated with the clinical SSI, with hepatomegaly, and with hemoglobin.
Osteopenia
Patients with GD are usually found to have osteopenia at all sites. It is typically manifested on radiographs as cortical thinning and may also be measured by dual-energy x-ray absorptiometry (DXA). Low levels of bone density in GD are associated with serum markers of accelerated bone turn over and breakdown, suggesting that the osteopenia of type 1 GD is associated with increased bone resorption.
The severity of osteopenia is related to overall disease severity and is correlated with genotype, the more severely affected N370S/84GG having lower density than milder N370S/N370S. As in individuals without GD, density typically declines as a function of age. DXA has been advocated for following response to therapy for children with GD, although absolute measurements do not correlate with severity of disease.
Unlike DXA and radiography, quantitative CT (QCT) can be used to detect the amount of reduction in purely trabecular bone. However, in patients with GD the observed reduction has been unimpressive. Presumably in patients with GD cortical bone loss predominates, suggesting that the role for quantitative CT is limited. In addition, when performed using conventional single-energy methods, replacement of normal marrow fat with Gaucher cells tends to artifactually elevate the apparent bone mass. Although rarely performed because of technical complexity, dual-energy CT measurements more accurately reflect the degree of bone loss.
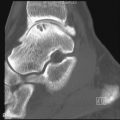
Focal Gaucher Deposits
Focal accumulation of Gaucher cells may result in osteolytic lesions ( Figure 27-3 ). These are typically asymptomatic unless infarction or fracture supervenes. Extraosseous GD may occur when focal marrow deposits grow so large that they violate the cortex. Such focal deposits, commonly referred to as “Gaucheromas,” might simulate malignancy ( Figure 27-4 ).


Gaucheromas are mass-like extensions of Gaucher marrow deposits into the soft tissues.
This differential may occasionally become problematic in view of the increased risk of multiple myeloma in patients with GD. In one rare instance, an extraosseous Gaucher cell accumulation even produced a monoclonal immunoglobulin (Ig) G kappa gammopathy, further simulating a myeloma. Gaucheromas tend to be very slowly progressive. In some instances the connection to the bone, although always present, may be subtle.
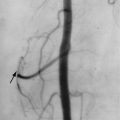
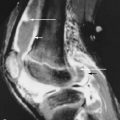
Osteonecrosis
Osteonecrosis is extremely common in patients with GD. As in unaffected individuals, it manifests in one of two clinical forms depending upon the anatomic site. Osteoarticular necrosis affects the ends of bone and leads to a clinical syndrome that usually results in collapse of the articular cortex and destruction of the adjacent joint ( Figure 27-5 ). Medullary necrosis affects the shaft or metaphysis of the bones. Medullary necrosis, although often painful, usually resolves without clinical sequelae other than residual imaging changes within the affected bones. Following an episode of infarction due to small vessel disease, the bone marrow appears diffusely sclerotic. If larger territories are affected, the infarcted area may appear as a focal lucency surrounded by a calcified or partially calcified margin. During an episode of acute pain caused by osteonecrosis, radiographs and CT may be normal, but MRI demonstrates bright signal within the affected marrow on T2-weighted images ( Figure 27-6 ). As in patients without GD, scintigraphy may reveal osteonecrosis as a focal area of diminished perfusion.