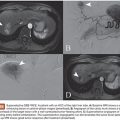
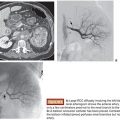
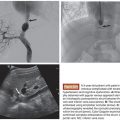
Miyuki Sone • Yasuaki Arai
Gelatin sponge (GS) has been used worldwide for more than 40 years for various embolization procedures. Although it was originally developed as a surgical hemostatic material in 1945,1,2 Ishimori et al.3 reported the first case of vascular embolization with GS for a carotid-cavernous fistula in 1967. Since then, GS has been a basic embolic material that is used in various disease entities such as hypervascular tumors, bleeding, and preoperative embolization.
DEVICE DESCRIPTION
General Characteristics
GS is prepared from purified porcine or bovine skin gelatin or collagen and processed with nitrogen to obtain a porous structure. It is biodegradable and insoluble in water. Although the commercially available products vary between countries, they are classified into two types based on their shape: preshaped and sheet-shaped. Preshaped GS (GELITA-SPON IR [Gelita Medical, Eberbach, Germany], Gelpart [Nippon Kayaku, Tokyo, Japan]) is a ready-to-use, 1- to 4-mm diameter dry product. Sheet-shaped dry GS (Gelfoam [Pharmacia & Upjohn, New York, USA], Sponzel [Astellas Pharma Inc., Tokyo, Japan], GELITA-SPON [Gelita Medical, Eberbach, Germany], Serescue [Nippon Kayaku, Tokyo, Japan]) requires manual preparation but is flexible in size and shape and can be used to treat various target vessels.
Mechanisms of Vessel Occlusion
Vessel occlusion with GS occurs mainly by mechanical obstruction caused by filling of the vascular lumen with GS and subsequent thrombus formation.4,5 Additionally, GS has hemostatic properties, which shorten coagulation time and accelerate thrombus formation.6
Recanalization and Resorption
GS is categorized as a temporary, degradable embolic material because of its unique features of resorption and vessel recanalization. The advantages of using degradable embolic materials are the preclusion of retaining foreign materials and recovery of blood flow in the target vessels. As a result, repeated embolization can be performed if required in the setting of various disease entities such as hepatocellular carcinoma or hemoptysis and for preserving fertility when embolization is used for the control of postpartum hemorrhage.
Because GS is insoluble in water, it is removed by phagocytosis, as a foreign body reaction usually occurs within 2 to 6 weeks.7 In animal studies, the time to vessel recanalization varies between a few days to several months.8,9 Louail et al.9 demonstrated that the recanalization rate of the porcine renal artery was 63% at 7 days and 100% at 14 days after GS embolization. In a similar study by Maeda et al.,10 the recanalization rate was 79% at 7 days after GS embolization. In a clinical study of uterine artery embolization (UAE) with GS by Katsumori et al.,11 magnetic resonance angiography performed at 4 months after UAE demonstrated recanalization of the ascending uterine artery in 88% of patients. However, there are several reports of recanalization failure in cases of preoperative embolization or embolization performed to control bleeding.12,13 Possible causes of inconsistency in the timing of recanalization after embolization include the amount of GS used, the inflammatory reactions induced, and the tissue necrosis that occurs after embolization. When the amount of GS is larger than the target vessel diameter, dense accumulation in the lumen may lead to an irreversible interruption of blood flow. An inflammatory reaction is also prominent after GS embolization.4,10,14 The inflammatory cells infiltrate the vessel wall, resulting in stenosis of the vessel because of the proliferation of fibroblasts, vasculitis, and acceleration of thrombosis.4,14,15 Furthermore, possible tissue necrosis after embolization may affect vessel patency10 because necrotic areas need fewer vessels than intact areas.
TECHNIQUE
Preparation of Sheet-Shaped Gelatin Sponge
To use sheet-shaped GS in vascular embolization, manual preparation is required to obtain the specific characteristics of the embolic material. Commonly used techniques include hand-cut, pumping, and torpedo.
Hand-Cut Method
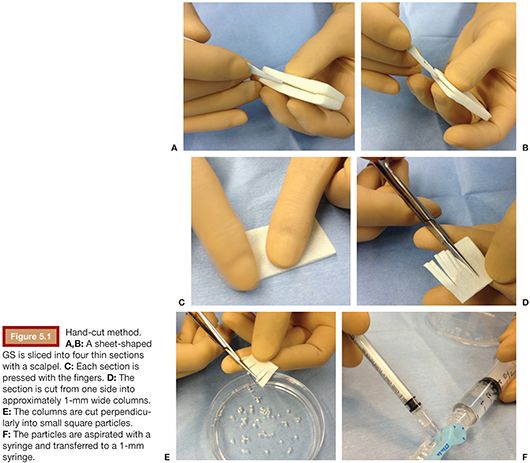
With this technique, sheet-shaped GS is sliced into four thin sections with a scalpel (Fig. 5.1A,B). Each section is pressed with the fingers or a Petri dish to make it thinner (Fig. 5.1C). The section is cut in half with a small straight scissors, which is then cut, starting from one side, into approximately 1-mm wide columns without cutting the end (Fig. 5.1D). Subsequently, every column is cut perpendicularly into small square particles and placed in a Petri dish filled with contrast material (Fig. 5.1E). The particles are aspirated with a syringe and transferred to a 1-mm syringe if a microcatheter is being used for embolization (Fig. 5.1F).
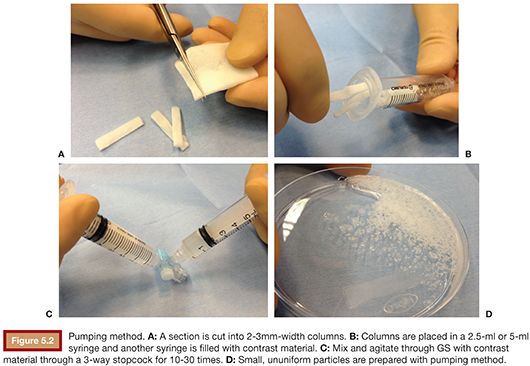
Pumping Method
Thin sections are prepared as described previously in the hand-cut technique. A section is then cut into 2- to 3-mm wide columns (Fig. 5.2A) and placed in a 2.5-mL or 5-mL syringe and another syringe is filled with contrast material (Fig. 5.2B,C). Subsequently, GS is agitated and mixed with contrast material through a three-way stopcock for 10 to 30 times (Fig. 5.2D).
Torpedo Method
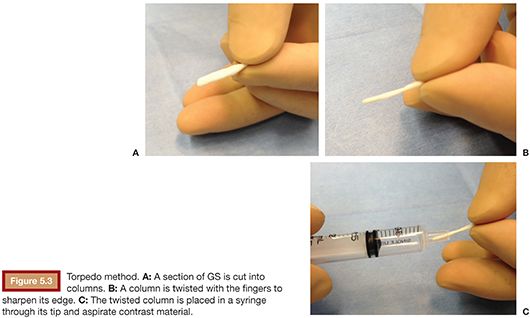
Thin sections are prepared as described in the hand-cut technique. A section is then cut into columns of the preferred size and a column is twisted with the fingers to sharpen its edge (Fig. 5.3A,B). These torpedoes are then placed in a syringe through its tip (Fig. 5.3C), and contrast is aspirated into the syringe as well.
Technical Considerations for Microcatheter Use
A 1-mL or 2.5-mL Luer Lock syringe is recommended when GS is injected through a microcatheter.16 In addition, the injection of normal saline between GS injections is recommended to avoid clogging of the microcatheter. Care should be taken when larger particles (>2 mm) are used because fragmentation can occur after the microcatheter is passed, resulting in very small particles and a more distal embolization than intended.17
Size and Distribution in the Vessels
The size of the GS is not uniform in both manually prepared GS sheets and preshaped GS.14,17,18 Specifically for the sheet-shaped GS, the size and uniformity of the particles largely depend on the preparation technique. Hand-cut GS is uniform in size, whereas the pumping technique produces particles with various sizes.18 Katsumori and Kasahara18 reported that cutting produced lower rates of smaller particles (≤500 μm) and larger particles (>2,000 μm) than pumping (8.5% vs. 20.4% and 0% vs. 48.1%, respectively) when Gelfoam was used.
Similar to other embolic materials, the vessel distribution after GS embolization depends on the size of GS. Although GS is deformable, it has the tendency to embolize vessels that are smaller than its size. Moreover, calibrating the size of GS particles is difficult not only for manually prepared sheet-shaped GS but also for preshaped 1-mm and 2-mm diameter GS.19 In theory, this represents the disadvantages of GS use, as the level of embolization is difficult to control. In animal studies, when 1- to 2-mm hand-cut and preshaped GS were used, the mean diameter of the occluded vessels was approximately 500 μm.14,20 In an animal study by Miyamoto,21 GS particles prepared by pumping were distributed in a wide range of porcine uterine arteries, including vessels less than 100 μm in diameter.
CLINICAL APPLICATIONS
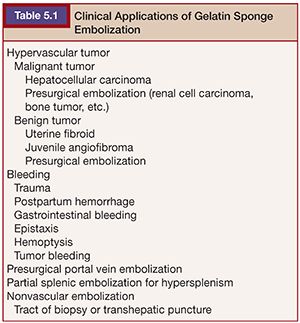
GS has been used extensively in various disease entities (Table 5.1). It has been used as antitumor treatment for hypervascular tumors and to control bleeding in various types of hemorrhages. Although data from randomized controlled comparative trials with newer spherical embolic materials are limited, the use of GS as a standard embolic material is supported by wide clinical experience and various clinical reports.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree