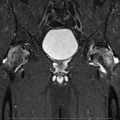
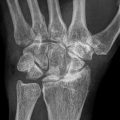
Fig. 9.1
Common pathway of developing osteonecrosis of the femoral head
9.2 Methods of Genetic Study
Various genetic studies had been developed and changed rapidly to identify disease-associated loci or to identify causal variation for specific disease. Single nucleotide polymorphisms (SNPs) are the most common form of DNA sequence variations. They can be very useful markers to map genes that modify the susceptibility of specific disease or those related drug responsiveness or side effect. Discovery of large numbers of SNPs is necessary to narrow down the genetic loci of specific disease susceptibility to small region of one gene by linkage disequilibrium mapping. Over the last few decades, genetic studies performing linkage analysis by positional cloning have identified about 3,000 genes as being inherited causing diseases. Linkage analysis which requires family pedigree is good for initial detection and, especially, is powerful for rare variants. To the contrary, candidate gene approach for association analysis was done to identify less than 1,000 SNPs using genotyping technology between a few hundred disease cases and controls under the concept of common variants implicated as common diseases. It is good for fine mapping, but poor for initial detection, and powerful for common variants.
Human genome project (HGP) identified 3,000,000,000 base pair DNA sequence. The reference sequence of genome constructed by the HGP is very informative about the vast majority of bases that are invariant across individuals. Subsequently, HapMap focusing on DNA sequence differences among individuals could inform substantial correlations of SNPs with many of their neighbors by recombination of linkage disequilibrium and low haplotype diversity. After international HapMap projects (Phase 1), genome-wide association study (GWAS) was used extensively through the world. GWAS enabled high-throughput genotyping more than 500,000 SNPs to identify disease-associated loci and rarely to identify causal variation of disease. It consisted of phenotyping (recruitment of thousands of cases and controls), genotyping of SNPs of individuals, and mapping to determine linkage disequilibrium block by statistical analysis. GWAS mapped a huge number of susceptibility genes for common diseases. However, because the proportion of heritability explained by GWAS was relatively low for common diseases, it had many false positives, and genetic markers applied in GWAS are common variants with minor allele frequency (MAF) greater than 5 % and odds ratio of phenotype less than 1.5. Genome-wide association meta-analysis (GWA MA) imputed 1.5–3 million genotyped SNPs. Next-generation sequencing (NGS) such as targeted resequencing for disease loci, whole-exome sequencing (WES) selective capturing and sequencing of all annotated protein-coding genes of whole exons, and whole-genome sequencing are expected to find to identify low-frequency (0.5 % < MAF <5 %) or rare (MAF <0.5 %) variants and high odds ratio for phenotype of disease in the near future(Table 9.1).
Table 9.1
Development of method in genomic research
Year | Method | Technology | Main purpose |
|---|---|---|---|
– | Linkage analysis | Positional cloning | Identification of rare variation for disease |
2005 | Candidate gene approach for association analysis | Genotyping (1–1,000 SNPs) | Identification of disease-associated loci |
Identification of causal variation for disease | |||
2007 | GWASa | High-throughput genotyping (>500 K SNPs) | Identification of disease-associated loci |
2011 | GWA MAb | Imputation/meta-analysis (>1.5–3 M imputed/genotyped SNPs) | Identification of causal variation for disease(rarely) |
2011 | Targeted resequencing for disease loci | NGSd | Identification of causal variation for disease |
2012 | Exome sequencing | ||
Future | WGSc |
9.3 Genetic Studies in Osteonecrosis of the Femoral Head
The cause of ONFH is multifactorial. Both genetic predisposition and exposure to certain environmental factors are associated. The incidence or prevalence of idiopathic ONFH reflects ethnic differences [16]. Some patients who have taken high dose of corticosteroids or excessive alcohol intake for long period develop ONFH, but rare cases of ONFH occur after short corticosteroid treatment indicating the presence of differences in susceptibility to risk factors as well as genetic predisposition to ONFH between individuals [17, 18]. Particularly, idiopathic ONFH in identical twins or a clustering of cases in families implicates involvement of genetic factors [19, 20].
9.3.1 Hypercoagulopathy and Genetic Alteration
Increased intravascular coagulation, hypercoagulability, seems to be associated with the development of ON [11]. The role of sickle cell disease and other hemoglobinopathies in the development of ONFH had been documented as undergoing the pathway of intravascular coagulation.
There were two studies using pedigrees linkage analysis followed by positional cloning on familial autosomal-dominant ONFH in Taiwanese. Chen et al. mapped the presence of mutations in the protein C, protein S, and PAI-1 protein to the 2q14-q14, 3q11.1-q11.2, and 7q21.3-q22 chromosomal segments, respectively. Liu et al. showed that the mutation in COL2A2 (mapped on chromosome 12q13) is associated with the genetic cause of ONFH. COL2A2 mutation, a major structural protein in the extracellular matrix of cartilage, was identified in three families with idiopathic ONFH showing autosomal-dominant inheritance [21].
Thrombophilia due to protein C and protein S deficiency was reported to have an association with ONFH. Pierre-Jacques et al. reported a familial heterozygous protein S deficiency in a patient with multifocal ON [22].
Factor V Leiden (G1691A, Arg506Gln) through generation of coagulation factor V results in a hypercogulable state. Homozygosity in the mutation is associated with much higher increase in the lifetime risk of venous thrombosis than heterozygosity in the mutation(100-fold versus 7-8 fold) [23].
Mutations in the factor V Leiden (FVL; G1691A) were significantly more common in patients with idiopathic ON than in a healthy population. Glueck et al. compared the prevalence of heterozygote for the FVL mutation between 244 ON patients (161 idiopathic and 83 secondary ON) and 104 controls [24]. Twenty-three of 244 patients with ON (9.4 %) had heterozygote for FVL, whereas 2 of 104 (1.9 %) controls had heterozygote for it. Fifteen of 161 patients with idiopathic ON (9.3 %) and 8 of 83 (9.6 %) patients with secondary ON had heterozygote for FVL. In contrast with this study, our study comparing the incidence of heterozygote in Korean population (423 patients with ON versus 348 controls), however, could not find significant difference (unpublished data). Therefore, the prevalence of the FVL mutation seems to be different in ethnic groups.
The substitution of G for A at nucleotide position 20210 in prothrombin gene (PTG) which results in increased plasma prothrombin levels has been reported to be associated with increased risk of thrombosis [25]. Almost all of studies reported negative association between G20210A and primary ON [26–30], only one reported the presence of G20210A associated with ON of knee with an OR of 3.6 compared with control subjects [31].
Plasminogen-activating inhibitor-1 (PAI-1) is a critical factor that regulates coagulation and fibrinolytic systems. Homozygosity or heterozygosity for 4G allele (4G/4G) has been reported to increase the levels of PAI-I levels, and this leads to reduced plasma fibrinolytic activity [29, 32]. PAI-1 gene is reported to be polymorphic, especially in rs1799889 (−675 4G/5G SNP) of the promoter region. Homozygosity for the 4G allele (4G/4G) has been reported to significantly increase the plasma PAI-1. Recently, Kim et al. reported prevalence of rs1799889 (−675; 4G/5G) and rs2227631 (−844G/A) in the promoter, and +107009 C/T in the 3′UTR (rs11178) SNP of PAI-1was significantly high in 206 patients with ONFH than that of 251 controls [33]. PAI-1 4G/5G polymorphism, particularly 4G allele, may be a risk factor for ON [29, 30, 33].
The 5,10-methylenetetrahydrofolate reductase (MTHFR) is the metabolic enzyme involved in the conversion of homocysteine to methionine via the remethylation pathway. Reduced levels of MTHFR activity lead to elevated plasma concentrations of homocysteine, a condition referred to as hyperhomocysteinemia. Hyperhomocysteinemia has also been identified as an independent risk factor for thrombotic events and ON [30]. Several case-control studies have examined the association between the C677T polymorphism in MTHFR and ONFH. However, reports concerning the role of this polymorphism in the pathogenesis ONFH have been inconsistent [29, 34, 35]. The association between the MTHFR C677T gene polymorphism and the development of ON was reported in a small number of Caucasian people [29, 34, 35]; we could not find any association between the MTHFR C677T gene polymorphism and the development of ON in 443 Korean patients with ONFH and 273 controls [35]. Recent meta-analysis with eight study samples reported that there was an association between MTHFR C677T polymorphism and ONFH in non-Asian population, but not for Asian population, although study samples were not considered etiology [36].
Tissue factor pathway inhibitor (TFPI) is an important regulator of blood coagulation pathway, and mutations of TFPI genes can increase the risk of thrombin generation and venous thrombosis. The haplotypes of AAAT and GAAT of TFPI were associated with the risk of ONFH in 474 Korean population with ONFH compared with 349 normal controls [37].
9.3.2 Genetic Risk Factors for Glucocorticoid-Induced Osteonecrosis
The high-dose corticosteroid use (~2 g of prednisone within 2–3 months) is the most common risk factor accounting for almost 10–30 % of ON cases [1, 38, 39]. However, only 8–10 % of patients exposed to corticosteroid therapy may develop ON [1]. Since not all patients who are treated with steroids develop ONFH, the presence of additional risk factors or individual variation of sensitivity for glucocorticoids has been suggested. However, no definite risk factor has been confirmed to date [40]. Genetic studies in subgroups of patients with steroid-induced ON first focused on coagulation, fibrinolytic factors, and homocysteine metabolism. Some authors reported a positive association of PAI and MTHFR [30] or factor V Leiden [31] polymorphisms with steroid-induced ON, but these studies included a relatively small number of patients. Moreover, results in these studies were not replicated in other studies [27, 34].
The 3435TT genotype and homozygosity in the mutant 2677T/A variants in the multidrug resistance gene 1(ABCB1; MDR1) had a protective effect against the development of ONFH after renal transplantation. These two polymorphisms were found to be in linkage dis-equilibrium [41]. The ABCB1 polymorphism was associated with ONFH in patients with SLE in Chinese people [42]. The 3435TT genotype individuals had increased pump activity of P-gp which might prevent the accumulation of steroids in bone cells. These results suggested genes related to steroid metabolism might influence sensitivity for steroid in patients with ONFH. However, polymorphisms in the CYP3A4, CYP2D6, and CYP2C19 isoforms and the CYP3A4 promoter region of cytochrome P450, participating in the drug metabolism of steroid, had not been associated with the increased risk of ON [43].
The development of ONFH increases in renal transplanted patients treated with glucocorticoids [43, 44]. Ferrari et al. reported a positive association of PAI 4G/4G genotype with ON [32]. However, other studies have not replicated this result [44, 45].
Recent meta-analysis showed there was no evidence for significant association between MTFHR C677T and ABCB1 G2677/A polymorphisms and risk of steroid-induced ON. However, the PAI-1 4G/5G and ABCB1 C3435T may be risk factors for steroid-induced ON.
9.3.3 Genetic Risk Factors for Alcohol-Induced Osteonecrosis
Liver alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH) converts ethanol to acetic acid. Acetaldehyde quickly forms free radicals which are highly toxic to the kidney and liver. The liver ADH, ALDH, and cytochrome P4502E1 are polymorphic at the ADH2, ADH3, ALDH2 loci, and the 5′-flank region of the P4502E1. Individuals with ADH2 2/2 genotypes were reported to have faster rates of alcohol metabolism. The ADH2*1 allele was lower in the ONFH patients than in the cirrhosis subgroup [46].
9.3.4 Other Genetic Abnormalities
In recent years, we have focused on new candidate genes participating in hypoxia, angiogenesis, adipogenesis, and osteogenesis for genetic association with ONFH.
A vascular hypothesis is considered to be the most persuasive in explaining pathogenesis of ONFH. It assumes that if thrombosis occurs, it is followed by a sequential process of blood flow obstruction, increased venous pressure, impaired arterial flow, osseous hypoxia, and bone death [27, 31], which appear to be important in ONFH development; hypoxia-inducible factor 1 (HIF1) [47], vascular endothelial growth factor (VEGF) [40, 48], annexin 6 [49], and catalase (CAT) [50] showed significant and meaningful association with risk of ONFH.
Hypoxia can induce both apoptosis as well as necrosis of cells and is associated with vascular disease. HIF1 is a master transcription regulator induced by hypoxia and critically acts in a wide range of cellular regulation processes including glycolysis, apoptosis, erythropoiesis, and angiogenesis [51]. We firstly examined association between polymorphisms of HIF1-α and ONFH using 384 ONFH patients and 237 normal controls. Our study suggested that genetic variations in HIF1 alpha (−89A/T, −20T > C, +3033C/T, +14539A > T, +22348C > T, +244413T > C) were associated with the risk of ONFH in 443 Korean population with ONFH compared with 273 normal controls [47].
Oxidative stress is one of the factors inducing vascular injury and apoptosis. Alcohol promotes the generation of ROS resulting in oxidative stress. Steroid-induced ON in the rabbit demonstrated that administration of steroids promotes to the development of oxidative stress and oxidative injury in bone tissue soon thereafter [52]. A number of polymorphisms in the CAT have been described as being associated with several diseases, such as osteoporosis, hypertension, diabetes mellitus, Alzheimer’s disease, and vitiligo. Six polymorphisms (−89A/T, −20T > C, +3033C/T, +14539A > T, +22348C > T) of CAT gene were associated with the risk of ONFH in 443 Korean population with ONFH compared with 273 normal controls [50]. These results suggest that oxidative stress may play an important role in the pathogenesis of ONFH.
Nitric oxide synthesized by eNOS has vasodilatory effects on vascular tone, inhibits platelet aggregation, and modulates smooth muscle proliferation [53]. Many studies have been carried out to determine the associations between genetic polymorphisms in the eNOS gene and vascular diseases, including coronary artery disease or myocardial infarction, hypertension, stroke, and renal diseases. A few studies have showed an association between eNOS polymorphisms and ONFH. Allele 4a of a VNTR polymorphism in intron 4 and T-786C polymorphism in the eNOS gene were associated with idiopathic ONFH [54, 55]. Furthermore, we also found that Asp258Asp (rs1549758) and Glu298Asp (rs1799983) polymorphisms of eNOS gene were significantly associated with the risk of ONFH in Korean SLE patients [56].
VEGF, a major inducer of angiogenesis, is important for bone formation processes, such as normal growth plate morphogenesis, including blood vessel invasion and cartilage remodeling [57], and it has also been implicated in bone repair [58]. During hypoxia, HIF binds to the hypoxia-responsive elements and the expression of VEGF can be induced. This leads to the stimulation of angiogenesis [59]. The VEGF gene was reported to be polymorphic, especially in the promoter region (−2578, −1154, etc.), the 5′-UTR (−634, −7), and the 3′-UTR (+936). Several studies have shown that polymorphisms within the 5′-UTR have led to differences in VEGF expression and that they could influence the etiology of a variety of pathological conditions such as diabetic retinopathy, prostate cancer, and breast cancer. Our study showed that the −634G > C polymorphism in the VEGF promoter was significantly associated with an increased susceptibility of ONFH in 317 Korean patients with ONFH compared with 497 normal controls. Lee et al. also found that −1154A/G in promoter was associated with 74 steroid-induced ONFH compared with 160 age- and gender-matched normal controls [40]. Additionally, Liu et al. reported the VEGF −634G/C polymorphism was associated with ONFH in 220 Chinese patients with ONFH compared with 220 normal controls [60]. Also, rs1485766 and rs3775203 SNPs of VEGF-C gene were associated with the risk of ONFH in 460 Korean patients with ONFH compared with 300 normal controls, especially in idiopathic and steroid-induced ON [61].
Annexins have been implicated in inhibition of coagulation and have been reported as major components of matrix vesicles. SNPs and haplotypes of annexin A2 were associated with sickle cell necrosis [5]. rs9324679, rs9324677, rs10037814, and rs11960458 SNPs of annexin A6 gene were associated with the risk of ONFH in 443 Korean population with ONFH compared with 273 normal controls [48].
Recently, Hirata et al. examined the differences of gene polymorphism frequencies of apolipoprotein B (ApoB) and apolipoprotein A1 (ApoA1), which are important proteins for lipid transport, as well as of lipid parameters, between ONFH cases and referent patients among those who were subjected to renal transplantation [62]. The results showed that a higher frequency of 7623TT or CT of the ApoB gene was observed in ONFH cases than in referent patients. Regarding lipid parameters, a higher value of ApoB/ApoA1 ratio was observed in cases. Miyanishi et al. also reported the relationship between ONFH and serum ApoB/ApoA1 ratio in a study of Japanese subjects [63].
Peroxisome proliferator-activated receptor-gamma (PPAR-γ) is predominantly expressed in adipose tissue. It regulates the lipid homeostasis and angiogenesis. −769 > G, +34C > G, and +82466c > T SNPs of PPAR-γ gene were associated with the risk of ONFH in 448 Korean population with ONFH compared with 336 normal controls [64].
SREBP-2 plays central role in the maintenance of lipid homeostasis through stimulating expression of genes associated with cholesterol biosynthetic pathway. rs2267439, rs2269567, rs1052717, and rs2267443 SNPs of SREBP-2 were significantly associated with the risk of ONFH in 443 Korean population with ONFH compared with 273 normal controls [65].
SREBP-1, sterol regulatory element-binding protein, activates genes regulating lipid biosynthesis. IVS7 + 117A > G SNPs of SREBP-1 genes were significantly associated with the risk of ONFH in 423 Korean population with ONFH compared with 348 normal controls, especially in male [15].
We investigate SNPs of other genes relating angiogenesis and hypoxia. rs1880669, rs2692695, and rs2718806 SNPs of transferring (TF) gene were significantly associated with the risk of ONFH in 460 Korean population with ONFH compared with 300 normal controls, especially in idiopathic ON. rs4309, rs4344, and rs4461142 SNPs of angiotensin-1-converting enzyme gene were associated with the risk of ONFH in 460 Korean population with ONFH compared with 300 normal controls, especially in steroid-induced ON. The most significant association with risk of ONFH was an SNP (rs2453839) of the insulin-like growth factor binding protein-3, especially in idiopathic and alcohol-induced ON. Two SNPs (rs6837735 and rs1870377) of kinase insert domain receptor and three SNPs (rs12573218, rs12358370, rs2269091) of neutrophilin 1 were associated with the reduction of ONFH risk [61].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree