This chapter provides an introduction to pediatric genitourinary imaging for the radiology resident. It will focus on diseases commonly encountered in or specific to children and congenital anomalies. Diseases more commonly seen in adults will not be covered extensively in this chapter.
The goal of this chapter is not only to describe genitourinary diseases, but also to convey a systematic approach for imaging evaluation of these entities. Most imaging of the genitourinary system starts with ultrasound, and imaging algorithms should always respect the “as low as reasonably achievable” (ALARA) principle.
Imaging Techniques
Ultrasound
In children, ultrasound is the first-line imaging modality for evaluation of the genitourinary system. It is readily available, making it easily accessible for fast and effective evaluation of the kidneys and urinary bladder. The lack of ionizing radiation makes this an optimal imaging tool in children. However, one of the disadvantages of ultrasound is the inability to acquire functional information. In addition, image quality is operator dependent, creating variability among examinations.
The parameters of imaging are optimized based on the age and size of the child. The kidneys and urinary bladder are imaged with the patient in the supine position [ Fig. 6.1 ]. Additional imaging in the prone position is routine in children because the kidneys are not obscured by bowel gas, and some children are more comfortable when prone and thus more compliant. Transducer choice in children is optimized to patient size, with high-frequency linear transducers commonly used for neonates and lower-frequency curved transducers used for older children [ Fig. 6.2 ].

Color Doppler imaging is used as an adjunct to grayscale imaging to provide information regarding movement and vascularity. This can be effective in delineating specific pathologies, such as the absence of a ureteral jet in a ureter obstructed by a stone or the absence of blood flow in a torsed testicle. Arterial and venous Doppler waveform analysis is helpful in evaluating entities such as renal artery stenosis (RAS) and renal vein thrombosis (RVT).
Prenatal ultrasound imaging can give valuable information about the kidneys and urinary bladder. This screening test provides the first indication that congenital genitourinary abnormalities may exist and allows for early diagnosis of diseases such as ureteropelvic junction obstruction (UPJO), bladder exstrophy, and multicystic dysplastic kidney (MCDK). Correlating postnatal imaging studies with prenatal imaging is very helpful in the diagnosis and workup of patients, and it should be done whenever possible. Most congenital anomalies are evaluated nonurgently in the first month of life. One exception is the case of suspected posterior urethral valves. In this setting, imaging evaluation and workup should be done in the hospital soon after birth [ Fig. 6.3 ].

Ultrasound contrast agents, commonly used in adults and internationally, are now available for use in children in the United States. Voiding urosonography is one potential use for contrast material.
Fluoroscopy
Voiding Cystourethrography
Voiding cystourethrography (VCUG) is a common imaging study to evaluate the genitourinary system in children. Unlike ultrasound, VCUG can assess anatomy as well as function. It is the best test to evaluate both reflux and lower urinary tract anatomy and function.
After sterile catheterization of the urinary bladder, contrast is instilled via gravity. An 8 French feeding tube is commonly used for catheterization because it is generally the appropriate diameter for a newborn urethra and has a radiopaque strip for easy visualization. While instilling contrast into the bladder, early filling images are obtained to assess for ureteroceles or other potential filling defects [ Fig. 6.4 ]. The expected bladder capacity is estimated based on the child’s age and is used as a guide for volume of contrast instillation. After the urinary bladder is adequately filled, bilateral oblique views of the urinary bladder are obtained to assess the vesicoureteral junctions. This is particularly useful in cases of vesicoureteral reflux (VUR), where it is important to document the insertion site of the ureter. In cases of VUR, an AP image of the abdomen centered at the kidneys showing the highest grade of reflux observed is standard. The international reflux grading system classifies reflux from mild (grade 1) to severe (grade 5) [ Fig. 6.5 ].


As implied by the name of the examination, all patients should void during the course of this study. This is vital not only in evaluation of the urethra, but also increase detection of reflux which may occur only during voiding. Because males are much more likely to have urethral abnormalities than girls, it is important to obtain dedicated lateral views of the entire male urethra, specifically to evaluate for the presence of posterior urethral valves. In children younger than 1 year, a cyclic examination is performed. This entails imaging the patient during three separate filling and voiding cycles. Studies have shown that this increases the sensitivity of the test for the detection of VUR. Postvoid images of the kidneys and ureters should also be obtained to assess for adequate drainage of refluxed contrast material, and thus evaluate for the possibility of concomitant obstruction. Another study that can be used to evaluate for VUR is a radionuclide cystogram (RNC), which is described later in the Nuclear Medicine section.
Retrograde Urethrogram
The retrograde urethrogram (RUG) is a contrast study that evaluates the appearance of the anterior urethra in males. The most urgent indication for an RUG is assessment for acute urethral injury after trauma. The urethra may be damaged from a straddle injury or in the setting of pelvic fracture(s). Patients present with blood per meatus and/or inability to void. An RUG is also used to assess for urethral stricture when patients present with a decreased urinary stream, difficulty with urination, or hesitancy. Strictures are commonly associated with prior trauma and, rarely, infections in children. An RUG is also commonly used to evaluate the postoperative urethra, such as after hypospadias or epispadias repair [ Fig. 6.6 ].

With the patient in a steep oblique position, a small catheter is inserted into the anterior urethra, and contrast is injected while the distal urethra is occluded. A Foley catheter balloon inflated within the fossa navicularis or external pressure from fingers or a Zipser clamp can prevent contrast from leaking during injection under pressure. This allows for optimal distension of the urethra to the level of the external sphincter, which is identified by its smooth, conical contour.
Genitogram
In patients with ambiguous genitalia, a careful physical examination of the perineum is performed. Depending on the appearance of the perineum, water-soluble contrast is subsequently instilled through the appropriate orifice(s) to determine the patient’s internal anatomy. This test is useful in studying specific clinical entities such as ambiguous genitalia (ie, distinguishing females with congenital adrenal hyperplasia [CAH] from males with severe hypospadias) or the cloacal malformation.
Nuclear Medicine
Nuclear medicine examinations are used in conjunction with ultrasound and VCUG in the workup of genitourinary anomalies, as these examinations have the advantage of providing functional information about the urinary tract. The RNC is a sensitive test for VUR. Cortical scintigraphy can evaluate for functional renal parenchyma. Diuretic scintigraphy is used in evaluation of urinary tract dilatation, specifically to distinguish obstructive versus nonobstructive causes.
Radionuclide Cystogram
Similar to VCUG, this study entails instilling contrast material into the urinary bladder (in this case, Technetium 99m pertechnetate) and performing dynamic imaging of the kidneys and urinary bladder during filling and voiding to evaluate for VUR [ Fig. 6.7 ]. With an RNC, reflux is graded 1 to 3 as opposed to the five-point international reflux study grading scale used for VCUGs. Although VCUGs provide better anatomic clarity, RNCs generally have a lower radiation dose, and perhaps, higher sensitivity. The indication for VCUG versus RNC varies by institution. It is common to first study patients for reflux with a VCUG, then perform follow-up studies with the lower-dose RNC study. Males should always first be studied by VCUG because only a VCUG provides imaging of the urethra during voiding.

Cortical Scintigraphy
Cortical scintigraphy determines the amount of functioning renal tissue. A cortical localizing agent, technetium-99m-dimercaptosuccinic acid (DMSA), is used for optimal visualization of the renal cortex. Uptake is seen in the renal cortex, with relative areas of photopenia in the renal medulla and sinus.
Indications for this examination include assessment for renal scar as a result of prior infections or chronic reflux [ Fig. 6.8 ]. It is also used in the setting of acute pyelonephritis for cortical localization of the infection. Acute pyelonephritis may appear as a focus of photopenia with no associated volume loss. Follow-up imaging 6 months after resolution of the infection can help evaluate for scarring, characterized by a focus of photopenia with associated volume loss.

Congenital anomalies, such as ectopic kidney, horseshoe kidney, or ectopic fused kidney, can be seen with cortical scintigraphy because the renal parenchyma and anatomic location can be identified [ Fig. 6.9 ]. Scintigraphy is useful in distinguishing MCDK from severe hydronephrosis because an MCDK has no functioning renal tissue.

Diuretic Renography
In contrast with cortical scintigraphy, renography evaluates urine formation and excretion by the kidney. Technetium-99m-mercaptoacetyltriglycine (MAG3) or technetium-99m-diethylene triamine pentaacetic acid (DTPA) may be used for this examination. MAG3 is the preferred agent, particularly in patients with impaired renal function, because it has a higher first-pass extraction.
This study is indicated to differentiate obstructive versus nonobstructive urinary tract dilatation. After administration of the tracer intravenously, imaging of the kidneys is performed to visualize cortical uptake, urine formation, and urine excretion. If urinary tract dilatation is identified in a renal collection system, a diuretic challenge is performed with the administration of furosemide (1 mg/kg, max 40 mg). The rate of urinary excretion from the renal pelvis and ureter after a diuretic challenge is calculated and compared with a normal standard, often the contralateral kidney. The time required for half of the tracer to pass the ureteropelvic junction (UPJ) is calculated ( ![]() ). A
). A ![]() of less than 10 minutes is considered normal, and a
of less than 10 minutes is considered normal, and a ![]() of greater than 20 minutes is considered abnormal. A
of greater than 20 minutes is considered abnormal. A ![]() between 10 and 20 minutes is deemed equivocal.
between 10 and 20 minutes is deemed equivocal.
In infants and young children, the diagnosis of obstructive versus nonobstructive urinary tract dilatation is difficult due to the high capacity of the renal pelvis and relatively low urine output. Therefore, evaluation of dynamic images is important. This allows assessment of the function of the kidney, evaluation of changes in hydronephrosis before and after diuretic administration, and visualization of the overall washout curve of the kidney [ Fig. 6.10 ].

Computed Tomography
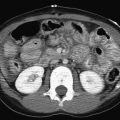
Computed tomography (CT) is readily accessible and images can be acquired quickly, reducing the need for sedation that may be required for MRI. However, CT should be used judiciously in children because it has the disadvantage of exposing patients to ionizing radiation. When performing CT scans in children, the ALARA principle should always apply. One should scan only when necessary, and pediatric protocols should be used. Low-dose pediatric protocols are now routine in pediatric hospitals, thereby decreasing the amount of ionizing radiation exposure per examination and the potential overall risk to children. Ultrasound, rather than CT, is the primary imaging modality for the urinary tract in children. However, CT is useful in certain clinical situations. Although ultrasound is the initial study of choice to evaluate for renal stones, noncontrast CT is useful when further clarification is needed [ Fig. 6.11 ]. Computed tomography angiography (CTA) and CT venography can provide exquisite detail regarding renal perfusion and drainage. Contrast-enhanced CT is useful in assessing the renal parenchyma, as well as in detecting underlying renal masses. Delayed postcontrast imaging demonstrates excretion of contrast material through the urinary system, highlighting the ureters and the urinary bladder. This may be particularly useful in the setting of trauma. Unlike the multiphase protocols frequently used in adults, a single imaging phase tailored to the specific clinical question typically suffices in children.

Magnetic Resonance Imaging
Aside from the specific indications for CT stated earlier, after ultrasound as the initial screening modality, magnetic resonance imaging (MRI) is typically the most commonly used cross-sectional examination for assessment of the genitourinary system. Similar to CT, MRI can show anatomic detail of the genitourinary system with the added ability to perform multiplanar acquisition. Additional advantages of MRI include lack of ionizing radiation exposure and superb soft tissue contrast resolution. One of the primary disadvantages of MRI is the long scan time, which necessitates sedation or anesthesia in some children. Pediatric patients with poor renal function should not receive intravenous gadolinium-based contrast agents because of the risk for nephrogenic systemic fibrosis, a rare disease that can affect the skin and various organs. In these patients, MR evaluation of the kidneys must be limited to noncontrast examinations.
Magnetic resonance urography (MRU) is a useful tool in evaluating the genitourinary system, both for anatomic detail and for functional information. Traditional T1- and T2-weighted imaging is performed, in addition to postcontrast sequences after the administration of intravenous gadolinium-based contrast material. This imaging technique not only helps in the assessment of the kidneys, urinary bladder, and vasculature, but also allows for the measurement of functional parameters of the kidney such as glomerular filtration rate and differential renal function.
Radiography
Radiographs are not typically performed as part of the initial imaging assessment of the kidneys and urinary bladder. However, pediatric patients may present with generalized, vague abdominal symptoms that may prompt clinicians to begin with radiographs. The KUB (kidneys, ureters, and bladder) provides a global view of the abdomen and is easily accessible and rapidly acquired.
Depending on the age and size of the patient, the outline of the normal renal shadows can be seen in the paraspinal region bilaterally at the L1-L2 level. Sometimes the renal shadows may be difficult to see secondary to overlying densities. Displacement of the normal bowel gas pattern by abnormal soft tissue may suggest the presence of an intraabdominal mass, including masses arising from the kidney. Radiopaque densities such as calcifications or renal stones may be best evaluated on radiographs. Although not always first obtained to evaluate the genitourinary system, radiographs often serve as a useful adjunct to other imaging modalities.
Normal Genitourinary System in Children
Kidneys
The imaging appearance of the kidneys is variable in children of different ages, with a typical pattern observed from the newborn period to adulthood. On ultrasound, the kidneys of infants typically have markedly hypoechoic medullary pyramids, and it is important not to mistake this finding for the dilated calyces of hydronephrosis. Over time, the echogenicity of the pyramids becomes more isoechoic to that of the surrounding cortex. Fetal lobation is seen in infancy, with the renal margins becoming smoother over time. It is notably possible to see remnant hypoechoic pyramids and fetal lobation even in teenagers [ Fig. 6.12 ].

Although the cortex of the adult kidney is normally hypoechoic compared with the liver and spleen, renal echogenicity is more variable in children and changes with age. In premature and term infants up to a few months of age, normal kidneys may be echogenic in comparison with the adjacent liver or spleen. Later in life, normal kidneys may be isoechoic or relatively hypoechoic to the adjacent viscera [ Fig. 6.13 ]. Loss of normal echogenicity and/or diminished corticomedullary differentiation are signs of medical renal disease.

As the child grows, so should the kidney. Charts are readily available to ensure that renal size is appropriate for the age the child. Renal growth should be relatively symmetric from side to side, with the left kidney typically slightly longer than the right. If the kidneys are larger or smaller than expected or if they are abnormally asymmetric in size, suspicion for underlying renal pathology should increase.
The normal CT and MRI appearance of pediatric kidneys, both with and without contrast, is very similar to that seen in adults. On T1-weighted images, the renal cortex is similar in signal intensity to the spleen, with the medullary pyramids relatively hypointense. On T2-weighted images, the renal parenchyma is overall hyperintense [ Fig. 6.14 ]. Fluid signal may be seen in the collecting systems and the ureters because of the presence of urine. Fetal lobations may be visible on these imaging modalities and should not be mistaken for renal scarring.

The renal collecting system is well profiled by a variety of imaging studies. In the past, intravenous pyelograms (IVPs) were frequently used. Today, CT and MRU have largely supplanted IVPs. In a normal, single-system kidney, 9 to 12 calyces should be present.
Ureters
The normal ureter is made up of musculature that allows peristalsis to promote antegrade flow of urine. On dynamic imaging, the normal, peristalsing ureter may be seen intermittently. It is more commonly seen when it is abnormally dilated. On ultrasound, a dilated ureter appears as a thin-walled, anechoic, tubular structure. Dilated ureters can typically be seen proximally at the level of the renal pelvis and distally in the deep pelvis. The ureteral insertion should be visualized to assess whether it is normal or ectopic. The middle portion of a dilated ureter may be difficult to see on ultrasound secondary to overlying bowel gas. A posterior approach may be helpful to bypass the bowel. Alternatively, a coronal approach using the kidney as an acoustic window may allow better visualization of the midureter. If there is concern for a possible ureteral abnormality that cannot be resolved by ultrasound the entire course of the ureters can be well seen on cross-sectional imaging. On MRI, for example, a dilated ureter appears as a T2 bright tubular structure that enhances on delayed postcontrast imaging.
Bladder
The normal urinary bladder is situated in the midline of the pelvis, just above the pubic symphysis and posterior to the rectus abdominous muscles. It is directly anterior to the uterus in females and anterior to the rectum in males. The bladder normally appears as a fluid-filled structure on all imaging modalities. The expected bladder capacity can be calculated based on the patient’s age, with older patients having larger bladder capacities.
The bladder wall should be thin but may become thickened in the setting of inflammation or hypertrophy from bladder outlet obstruction. On postvoid images or studies performed with a partially distended bladder, the wall may appear thickened because of redundant, collapsed tissue.
Urethra
The female urethra is shorter than the male urethra. The longer male urethra is anatomically divided into the anterior and posterior urethra. The anterior urethra is made up of penile and bulbar segments, whereas the posterior urethra is made up of membranous and prostatic portions. The prostatic urethra passes through the prostate, and the membranous urethra passes through the urogenital diaphragm.
The verumontanum is a mound of tissue along the posterior wall of the prostatic urethra [ Fig. 6.15 ]. This mound contains the orifices of three different structures: (1) the midline prostatic utricle, a Müllerian duct remnant; (2) paired ejaculatory ducts; and 3) the many ducts of the prostate gland. Posterior urethral valves are located at the base of the verumontanum.

The male urethra may be evaluated by both the RUG and the VCUG. The RUG evaluates only the anterior urethra and is useful for revealing strictures or disruption. Both the anterior and the posterior urethra are opacified during a VCUG, and this is the only test to reveal the presence of posterior urethral valves.
Congenital Anomalies of the Kidneys and Ureters
Embryology
Although an in-depth discussion of the embryology of the genitourinary system is beyond the scope of this text, certain points of embryogenesis are important to understand in appreciating the development of congenital anomalies. The kidney progenitor, the pronephros, is a transitory structure which develops in the fourth week of gestation and involutes during the fifth week. From this structure, the mesonephros arises and forms the primitive kidney and the mesonephric duct. While the primitive kidney involutes, the mesonephric or Wolffian duct persists. Its final form differs based on the sex of the developing fetus. In both sexes, the mesonephric duct forms a portion of the bladder and ureter (discussed later). In females, the mesonephric duct otherwise involutes, whereas in males, it is responsible for the development of the seminal vesicles, epididymis, and vas deferens.
The final form of the kidney arises as a bud from the mesonephric duct. This ureteric bud or diverticulum interacts with the metanephric blastema to form the kidney. The ureteric bud develops into the renal collecting system. The metanephric blastema forms the renal parenchyma.
The transformation from the pronephros into the final form of the kidney occurs in the pelvis. By the ninth week of gestation, the kidneys ascend to their typical position in the retroperitoneum [ Fig. 6.16 ].

Renal Anomalies
Abnormalities of Development, Ascent, and Fusion
During any of the earlier developmental steps, a mishap may occur resulting in a specific congenital anomaly. If the ureteric bud fails to develop off the mesonephric duct, renal agenesis may occur. Although unilateral renal agenesis may occur in a number of syndromes as well as sporadically, it is generally a benign entity. In females, there is a frequent association with Müllerian duct abnormalities, which will be discussed in a subsequent section of this chapter called Anomalies of the Female Genital Tract . Bilateral renal agenesis is incompatible with life due to the severe oligohydramnios and pulmonary hypoplasia that will ensue. In utero, development of the lungs is inextricably linked with development of the kidneys. Normal lung development depends on the presence of adequate amniotic fluid, which is mainly composed of fetal urine produced by the kidneys. Therefore, if fetal renal function is significantly impaired, there will be insufficient amniotic fluid production for normal lung development.
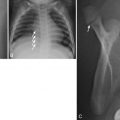
If the kidney fails to ascend appropriately, it remains in the pelvis as an ectopic pelvic kidney. Rarely, the kidney may ascend higher than normal, producing a thoracic kidney. This is a benign entity, but may be a source of confusion and concern on a chest radiograph.
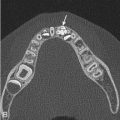
If there is abnormal coalition of the metanephric blastema, fusion of the kidneys will occur. When the lower poles of the kidneys are fused in the midline, they are unable to ascend properly and a horseshoe kidney results [see Fig. 6.9 ]. The band of parenchymal tissue joining the lower poles is referred to as the isthmus. This anomaly is frequently an incidental finding on imaging studies. However, horseshoe kidneys are predisposed to urinary stasis, stone formation, and injury in the setting of relatively minor trauma [ Fig. 6.17 ]. Whether horseshoe kidneys are predisposed to the development of malignancy remains a point of debate.

If the metanephric blastemas fuse off midline, cross-fused renal ectopia will result. There are a variety of manifestations of cross-fused renal ectopia, depending on both where the kidneys fuse and how far superiorly the fused renal mass is able to ascend [ Fig. 6.18 ]. This condition is frequently an incidental finding, and there are no deleterious effects on renal function. If surgery is planned in the setting of a fusion anomaly, close attention should be paid to the arterial supply and venous drainage because these are frequently aberrant.

Multicystic Dysplastic Kidney
An MCDK is a dysplastic, nonfunctioning kidney that is composed of multiple cysts. These cysts involute over time leaving a tiny soft tissue remnant. MCDK may present prenatally as a complex cystic structure in the expected location of the kidney, and may present postnatally as an abdominal mass. Although this condition typically involves the entirety of the kidney, segmental forms of MCDK can be seen.
Two different mechanisms have been proposed for the development of this condition. The first is related to abnormal induction of the metanephric blastema by the ureteric bud, with abnormal development of the renal parenchyma. The second involves atresia of the renal pelvis or proximal ureter early in the course of gestation, resulting in severe obstruction of the developing kidney.
On imaging studies, it is important to differentiate a severely hydronephrotic kidney from an MCDK. Whereas a hydronephrotic kidney may regain some function, an MCDK will not. This differentiation is made by determining whether the fluid-filled structures observed within the kidney communicate with one another. With hydronephrosis, the “cysts” (representing the severely dilated calyces) communicate, whereas with MCDK, they do not. Ultrasound cine clips are vital to assess this relationship as a single, static image may be misleading.
If the MCDK appears atypical or does not involute, a DMSA scan may be performed. There should be no cortical uptake of radiotracer by an MCDK, whereas faint radiotracer uptake will be seen in even a severely hydronephrotic kidney [ Fig. 6.19 ].

Abnormalities of the Ureters
Ureteropelvic Junction and Ureterovesical Junction Obstruction
Congenital obstruction of the ureter occurs most frequently at the UPJ and ureterovesical junction (UVJ). Congenital UPJO is postulated to most commonly result from abnormal development of the upper segment of the ureter, resulting in intrinsic narrowing. External compression from a crossing vessel is also a cause, although rare. Patients with intrinsic obstruction tend to present earlier. The fixed obstruction that causes hydronephrosis is often first detected on prenatal ultrasound. Patients with a crossing vessel have intermittent obstruction, and tend to present later in childhood or adolescence with recurrent abdominal pain. The kidneys are hydronephrotic only during episodes of obstruction, and frequently appear normal otherwise. Imaging should be done during the painful obstructive crisis to visualize hydronephrosis.
Prenatally, UPJO may present with hydronephrosis. Postnatally, if the obstruction is severe enough, it can present as a palpable abdominal mass because of the markedly dilated renal pelvis. Older children and even adults may present with intermittent abdominal or flank pain, recurrent infections, or hypertension. The diagnosis is most frequently made with ultrasound. A dilated renal pelvis and hydronephrosis are seen with a normal-caliber distal ureter [ Fig. 6.20 ]. Diuretic renal scintigraphy with MAG3 can determine whether there is a true obstruction. Depending on clinician preference, cross-sectional imaging with CT or MR may be performed to assess for a crossing vessel as the cause of obstruction. Recently, increased attention has been paid to dynamic MRU as a tool to help determine need for surgery. Treatment for this condition is typically with pyeloplasty, with resection of the intrinsically narrowed segment of ureter and creation of an anastomosis between the pelvis and ureter.

More rarely, congenital UVJ obstruction (UVJO) may occur. In a single-system kidney, this is most commonly due to primary megaureter. In this condition, a functional obstruction of the distal ureter prevents the normal egress of urine from the ureter into the bladder. This results in hydroureteronephrosis. Greater than 70% of cases of primary megaureter improve with time. Thus patients rarely require surgery. Although the ureter in UVJO typically inserts normally into the bladder at the trigone, UVJO may also be associated with ectopic sites of ureteral insertion. This is the case both for a single system and for the upper pole ureter of a duplex system.
Other forms of obstruction may occur anywhere along the length of the ureter. These are even rarer than UVJO. A midureteral valve may be seen, or a retrocaval ureter can be present with the right ureter coursing posterior to the inferior vena cava (IVC) (preureteral vena cava). This latter anomaly causes medial displacement of the ureter on urographic studies such as an IVP or MRU.
Vesicoureteral Reflux
Another congenital anomaly of the UVJ is VUR, thought to occur because of an abnormal width and length of the distal ureter as it inserts into the bladder wall. With this abnormality, the normally competent UVJ fails, allowing retrograde movement of urine from the urinary bladder up the ureter. This is thought to damage the kidney over time, predominantly because of recurrent infections. The extent of damage to the kidneys is directly related to the degree of reflux. VUR is often found during the evaluation of infections or prenatal hydronephrosis, but it may also be clinically silent.
VUR is most commonly detected and graded by two different imaging modalities: the VCUG and the RNC. In the United States the VCUG is the most commonly used modality. This technique is described in the Imaging Techniques section earlier in this chapter. Occasionally, there is concomitant reflux and obstruction, either at the level of the UPJ or the UVJ. If there is coexisting reflux and obstruction, reflux cannot be graded using the standard system. Refluxed contrast material will appear diluted when it enters a dilated, obstructed collecting system because it mixes with trapped urine. On delayed images, there will be prolonged retention of contrast material proximal to the obstruction, most commonly at the level of a UPJO. Thus delayed images are important for making the diagnosis of reflux coexisting with obstruction [ Fig. 6.21 ].

The treatment for reflux varies widely depending on the age of the patient, the severity of the reflux, and the preference of the individual practitioner. Therapeutic options include watchful waiting with antibiotic prophylaxis and surgery. Low-grade reflux in the infant and young child typically can be observed. As the patient matures and grows, the UVJ most often becomes competent and reflux resolves. For refractory cases, surgical reimplantation of the ureters or submucosal injection of a synthetic material (such as Deflux by Salix Pharmaceuticals) below the ureteral orifice is offered. The surgically created Deflux mounds may seem similar to soft tissue masses within the bladder and should not be confused for malignancy. Deflux may occasionally calcify. At the UVJ, this may mimic distal ureteral calculi. Knowledge of a history of Deflux injection is helpful to avoid diagnostic confusion [ Fig. 6.22 ].

Duplicated Collecting Systems
A duplicated collecting system results from either a bifid ureteric bud that arises from the mesonephric duct or two separate buds that meet the metanephros. Duplex collecting systems exist along a spectrum. At one end of the spectrum, just the renal pelvis may be bifid, with a single ureter exiting the kidney. This is frequently an incidental finding with no clinical significance. At the other end of the spectrum, a complete duplication may be seen, with two distinct renal moieties and separate ureters that insert at different locations in the urinary bladder.
When there is complete ureteral duplication, the upper pole ureter is abnormal and inserts medial and inferior to the orthotopic orifice. The lower pole ureter is the analog of the single-system ureter and inserts normally. This relationship was originally described by pathologists Weigert and Meyer, giving us the Weigert-Meyer rule .
While the insertion site of the upper pole ureter is always medial and inferior to that of the normal lower pole ureter, the exact location of the insertion varies. It may insert into the bladder proper, potentially extending inferiorly to the level of the bladder sphincter. In females, the insertion site may be below the urinary sphincter, into the distal urethra, vagina, or perineum. In males, the insertion site may include the urethra, but it is always proximal to the external sphincter and the Wolffian duct derivatives. The morphology of the upper pole ureteral insertion may also be abnormal, which can predispose to obstruction and reflux. The upper pole ectopic ureter may end in a ureterocele, a common cause of obstruction. A ureterocele is a dilation of the intravesical portion of the distal ureter, and it is seen as a thin-walled, ovoid structure protruding into the bladder lumen [see Fig. 6.4 ]. Ureteroceles seen in association with the upper pole of a duplex kidney are more common in females. They may also be seen in single-system kidneys, more commonly in males.
A duplicated system is most frequently initially detected with ultrasonography. The presence of a duplicated collecting system is suggested when a band of normal renal parenchyma is seen bifurcating the renal sinus fat [ Fig. 6.23 ]. If the duplex kidney appears otherwise normal, no further evaluation is needed. An incomplete ureteral duplication is likely present.

When imaging findings suggest that the upper and lower poles of a duplex kidney are functioning independently, a complete duplication is likely. An example of this is lower pole hydronephrosis with a normal-appearing upper pole. The presence of a ureterocele, particularly in a female, is also suggestive of a duplicated collecting system.
Patients with suspicion of complete ureteral duplication should undergo further evaluation with a VCUG. RNC has no role in the initial workup of these patients because it provides insufficient anatomic detail. A ureterocele is best detected with a minimum amount of contrast material in the bladder and appears as an ovoid filling defect [see Fig. 6.4 ]. Because of its thin wall, a ureterocele can collapse or efface once the bladder is full. It may also evert or intussuscept, mimicking a bladder diverticulum. Thus early filling views are essential for accurate detection.
A careful evaluation for reflux should be performed. Lower pole reflux is a frequent finding in complete ureteral duplication. This may be mistaken for reflux into a single system, particularly if there is no prior imaging such as ultrasound for comparison. Certain characteristics of the opacified collecting system can be helpful in differentiating reflux into the lower pole of a duplex system from reflux into a single-system kidney. In a single-system kidney, when the refluxed contrast extends to the level of the collecting system, 9 to 12 calyces should fill, and the longitudinal orientation of the calyces should point toward the contralateral shoulder. If contrast fills a smaller number of calyces and the longitudinal axis of the kidney points toward the ipsilateral shoulder [ Fig. 6.24 ], the presence of a duplicated collecting system with lower pole reflux should be considered. The appearance of lower pole reflux has been compared with that of a “drooping lily” [ Fig. 6.25 ]. This appearance is related to mass effect from a hydronephrotic upper pole system and the orientation of the calyces (petals) toward the ipsilateral shoulder. Reflux can also rarely occur into the upper pole of a duplex system.


Functional imaging, either by nuclear medicine or MRU, is useful in assessing the degree of function of the upper and lower poles, and also in the evaluation for obstruction. Typically, the more ectopic the ureter, the more dysplastic and dysfunctional the associated upper pole. The lower pole may have decreased function or scarring from dysplasia or reflux.
The clinical presentation of a duplicated collecting system can vary widely. Most frequently, particularly in the case of an incompletely duplicated system, there are no clinical ramifications. With a completely duplicated system, patients may present with recurrent infections because of reflux or obstruction. Hydronephrosis may also be discovered, also related to reflux or obstruction. Obstruction will typically involve the upper pole.
A duplicated collecting system with an ectopic upper pole ureteral insertion can be a cause of incontinence in a female. Males do not experience this type of incontinence, because the ectopic ureter always inserts above the external urinary sphincter. As discussed earlier, in females the ectopic ureter may insert below the level of the sphincter, either into the urethra or the vagina. With these forms of ectopic insertion, there is constant leakage of urine. Thus an ectopic ureter causing incontinence may be diagnosed with the clinical history of a girl who has never been dry, day or night. Physical examination will show dribbling of urine at the perineum. In this setting, the goal of the radiologist is to find the offending upper pole ureter [see Fig. 6.14 ].
Congenital Anomalies of the Bladder and Urethra
Embryology
While the kidneys and ureters arise from the ureteric bud, the bladder and urethra arise predominantly from the cloaca. Early in fetal life there is a single orifice for the bladder, rectum, and in females, the vagina. This is the cloaca. By the sixth week of gestation, a urorectal septum develops, dividing the cloaca into the urogenital sinus ventrally and the rectum dorsally. The cephalic portion of the urogenital sinus unites with the caudal end of the mesonephric duct, and together these structures form the bladder and ureteral orifices. The caudal portion of the urogenital sinus forms the urethra in males, and both the urethra and the vagina in females [see Fig. 6.16 ].
Congenital Anomalies of the Bladder
Urachal Anomalies
The allantois is a structure that drains the fetal bladder during the first trimester. It extends from the bladder dome to the umbilicus. In most cases it eventually involutes, leaving the urachus or median umbilical ligament. Portions of the duct may remain patent postnatally. When the entirety of the duct remains open, a patent urachus results. This presents with drainage of urine through the umbilicus. A blind ending pouch extending inferiorly from the umbilicus is a urachal sinus, which can present with infection. A portion of the urachus that remains fluid filled with both ends closed is a urachal cyst. This entity may present with infection or, if large enough, a palpable abdominal mass. Finally, the urachus may remain open at the level of the bladder dome, resulting in a urachal diverticulum. Urachal anomalies may be visualized with ultrasound and sometimes with cystography (both CT and fluoroscopic) as fluid-filled tubular structures following the expected trajectory of the median umbilical ligament.
Neurogenic Bladder
Neurogenic bladder, although not a congenital anomaly resulting from maldevelopment of the bladder or urethra, is an anomaly that can be present at birth secondary to an abnormality of the spinal cord such as myelomeningocele or tethered cord. In the setting of such an abnormality, the bladder does not receive appropriate innervation, and two different problems may result. The bladder sphincter may be weak, leading to incontinence and a low capacitance bladder. The bladder wall will be of normal thickness. Alternatively, bladder sphincter dyssynergia may result, where the bladder contracts but the sphincter does not relax. This results in marked hypertrophy and trabeculation of the bladder wall. The thickened, trabeculated, elongated appearance of the bladder on VCUG in this setting has been likened to that of a pinecone or Christmas tree [ Fig. 6.26 ].

Management for these patients includes intermittent catheterization to empty the bladder completely and regularly, as well as anticholinergics to relax the bladder wall. Not infrequently, however, the bladder may need to be surgically augmented. Augmentation is most commonly performed using ileum, followed by other segments of bowel including colon or stomach. The classic configuration of an augmented bladder is a hollow viscous with the appearance of the bladder inferiorly and bowel segment superiorly. Often the original valvulae conniventes or haustra can be appreciated in the augmented section.
Bladder Diverticula
Bladder diverticula can be seen in patients of all ages. The younger the patient, the more likely the diverticulum is to be congenital in etiology. Congenital diverticula are due to abnormalities of the detrusor muscle of the bladder wall, allowing for outpouching of the urothelium through a muscular defect. Congenital diverticula frequently occur near the ureteral orifice, creating a periureteral or Hutch diverticulum. Acquired diverticula may be multiple and occur most frequently in the setting of bladder outlet obstruction because of increased intraluminal pressure. They may occur in patients with a neurogenic bladder (discussed earlier). Rarely, multiple bladder diverticula may be seen in Menkes kinky hair syndrome, Williams syndrome, or Ehlers-Danlos syndrome.
Abnormalities of the Urethra
Posterior Urethral Valves
Continuing the march down the urinary tract, we will end our discussion of congenital anomalies with the urethra. Congenital bladder outlet obstruction is seen predominantly in males due to posterior urethral valves. Although the exact embryologic mechanism responsible for the development of valves remains a point of debate, they result from abnormality in the formation of the urethra. Posterior urethral valves are overwhelmingly the most common form, although anterior urethral valves may also exist and cause obstruction. With advances in fetal imaging, posterior urethral valves are frequently suspected before birth. Postnatally, patients may present with recurrent infections and hydronephrosis in more mild forms of PUV, or urosepsis in more severe forms. If not diagnosed early in life, patients are at risk for development of renal failure caused by chronic obstructive uropathy.
Prenatal ultrasonography may demonstrate a dilated, thick-walled urinary bladder with a keyhole appearance, representing the dilated urinary bladder and posterior urethra [see Fig. 6.3 ]. Hydronephrosis or hydroureteronephrosis is a frequent coexisting finding due to obstruction and/or reflux. The degree of dilation is typically asymmetric between the right and left sides. If the obstruction causes forniceal rupture, urinomas may form or urinary ascites may be seen. In severe cases, PUV causes decreased urine production, oligohydramnios, and pulmonary hypoplasia. On postnatal ultrasound, one may see a dilated, thick-walled urinary bladder with a variable presence and degree of hydroureteronephrosis.
Although findings on ultrasound may be suggestive of PUV, the diagnosis ultimately depends on lateral voiding images of the urethra obtained during a VCUG. Valves are seen as thin slips of tissue and are located at the base of the verumontanum (discussed in the earlier section of this chapter called Normal Genitourinary System in Children .) Because the valves are anteriorly positioned within the urethral lumen, the stream of contrast will be eccentrically positioned along the posterior aspect of the urethra. The urethra will be dilated proximal to the valves. The degree of proximal urethral dilation, bladder wall thickening, and reflux depend on the severity of the valves [see Fig. 6.3 ]. Treatment for this condition is surgical endoscopic fulguration of the valves.
Other Congenital Anomalies of the Genitourinary System
Anomalies with a short urethra and abnormal location of the urethral meatus include hypospadias and epispadias. The meatus is located on the ventral surface of the urethra in hypospadias, and along the dorsal surface in epispadias. Hypospadias occurs only in boys, whereas epispadias occurs in both boys and girls.
Hypospadias
Hypospadias occurs because of abnormal development of the urethral groove. The degree of hypospadias may vary widely, with the site of urethral exit seen anywhere from immediately inferior to the expected position to a more proximal position along the penile shaft to the anal verge. The prostatic utricle, a midline diverticular structure arising from the verumontanum, is a normal Müllerian duct remnant that becomes enlarged in patients with hypospadias. The more severe the hypospadias, the larger the associated prostatic utricle. Hypospadias most frequently occurs as a sporadic, isolated malformation, but it may occur in constellation with other abnormalities such as cryptorchidism or imperforate anus. Hypospadias is diagnosed on physical examination. Preoperative VCUG is useful to evaluate for the presence of a refluxing prostatic utricle that may require excision. RUG is helpful postoperatively to assess for complications. In severe hypospadias, the dysmorphic physical appearance is on the spectrum of ambiguous genitalia. The distinction between severe hypospadias and other forms of ambiguous genitalia, such as CAH, is made based on a combination of radiologic findings (ultrasound and genitogram), physical examination, and laboratory data.
Exstrophy-Epispadias Complex
Epispadias is secondary to failure of midline fusion of the urethra, a problem occurring much earlier in embryogenesis than hypospadias. It is a part of the continuum of the exstrophy-epispadias complex. In patients with complete bladder exstrophy, the normally spherical bladder forms instead as a flat plate, a process termed detubularization . The bladder mucosa is exposed on the anterior surface of the abdominal wall. The urethra is similarly detubularized and forms a urethral plate. When only the urethra is detubularized, the result is epispadias. There are many associated anomalies. One is diastasis of the pubic symphysis, which occurs because of outward rotation of the pelvis and bony deficiency, as well as a change in the pelvic floor muscular configuration [ Fig. 6.27 ]. The genitalia also have an abnormal appearance, with a bifid clitoris in females and separation of the corporal tissue in boys, which is mild in epispadias and more severe in exstrophy. In patients with bladder exstrophy, the ureteral insertions are abnormal. Once the bladder is closed, VUR is nearly universal.

Epispadias is suspected on prenatal imaging by ambiguous genitalia, with the phallus typically much shorter than normal in males. It may be occult in females. Bladder exstrophy is a more obvious diagnosis on prenatal imaging and is considered when one does not visualize the normal fluid-filled bladder consistently during the course of the examination. Other findings suggestive of the diagnosis include widening of the pubic symphysis and visualization of an abnormal mass reflecting the bladder plate along the anterior wall of the pelvis.
Postnatally, the diagnosis of epispadias in boys and bladder exstrophy in boys and girls is clinically obvious. The role of imaging, if any, is primarily for surgical planning. Epispadias is often missed in females because the only visible sign on physical examination may be a bifid clitoris. MRI is useful in evaluating the pelvic musculature, bladder plate, and pubic diastasis in patients with bladder exstrophy. After the bladder has been reconstructed and the epispadias repaired, cystography is frequently performed to assess the size and integrity of the bladder and urethra. Ultrasound is used to monitor the appearance of the entire genitourinary system, particularly the kidneys, which are prone to scarring from reflux.
Prune Belly Syndrome
Prune belly, or Eagle Barrett syndrome, is a rare disorder characterized by the presence of three major findings: marked dilatation of the ureters, cryptorchidism, and significant deficiency of the abdominal musculature. Abdominal wall muscular deficiency is responsible for the characteristic physical examination finding of a wrinkled or prunelike belly. The etiology of this condition is unknown, but it is thought to be due to abnormalities of the mesenchyme early in gestation. The developing fetus is unable to expel urine appropriately, resulting in obstructive uropathy, maldeveloped kidneys, oligohydramnios, and pulmonary hypoplasia.
Early postnatal radiographs of affected infants will have an unusual appearance. The abdominal contents will be splayed laterally because of the lack of normal musculature. Ultrasound will show markedly dilated ureters, associated hydronephrosis, and a dilated urinary bladder. The kidneys are typically dysplastic and appear echogenic. Ultrasound can also aid in the diagnosis of cryptorchidism. With VCUG, one will see an enlarged urinary bladder with reflux up massively enlarged, tortuous ureters into dilated renal collecting systems. A urachal diverticulum is a frequent coexisting finding. The posterior urethra is typically dilated due to deficiency of the prostate gland. Occasionally, the anterior urethra is dilated (“megalourethra”).
The prognosis for these patients greatly depends on the degree of urinary tract abnormalities and pulmonary hypoplasia. In terms of genitourinary care, patients frequently undergo clean intermittent catheterization or vesicostomy to improve the egress of urine, decompress the urinary system, and avoid further injury to the kidneys. Patients are prescribed antibiotic prophylaxis because of the severity of VUR.
Acquired Abnormalities of the Genitourinary System
Infections of the Genitourinary System
Urinary tract infections (UTIs) are very common in children, surpassed only in frequency by upper respiratory tract infections. The term UTI generally refers to an infection anywhere along the urinary tract. Pyelonephritis is distinguished from cystitis by high fever (>101.3°F), systemic symptoms, and flank pain.
The clinical presentation of an infant with a UTI is nonspecific but typically includes fever and irritability. As part of the workup for a fever without a clear source, a urine sample is often obtained. Ideally, a catheterized specimen is collected in non-toilet-trained children, and a clean catch specimen is obtained in toilet-trained children. This minimizes the risk for contamination. The diagnosis is made when a single organism grows more than 50,000 colony-forming units. The clinician must then decide whether to pursue further imaging to determine the cause of the UTI. One approach is to routinely perform both renal ultrasound and VCUG after the initial infection. The purpose of these studies is to assess for the presence of VUR (discussed earlier in this chapter). Recommendations from the American Academy of Pediatrics in 2011 state that a renal ultrasound should be performed after the initial infection in children aged 2 to 24 months, but that a VCUG need not be performed unless there are abnormalities on ultrasound or the patient experiences recurrent febrile UTIs. That said, many studies have shown that a normal ultrasound does not exclude the presence of VUR, even high grade, and is a poor screening test for reflux.
Pyelonephritis
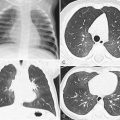
When infected, the kidney may appear enlarged with loss of corticomedullary differentiation. With power Doppler, regions of relative hypoperfusion may be demonstrated [ Fig. 6.28 ]. On CT or MRI, the kidney is noted to be enlarged, with variable amounts of perinephric fluid. On postcontrast images, a “striated nephrogram” pattern may be observed. Poorly defined regions of hypoenhancement may also be seen. On a DMSA study, regions of diminished uptake are noted. These can remain for weeks to months after the infection, and may reflect areas of scarring if they persist.