BASIC ANATOMY OF THE URINARY SYSTEM
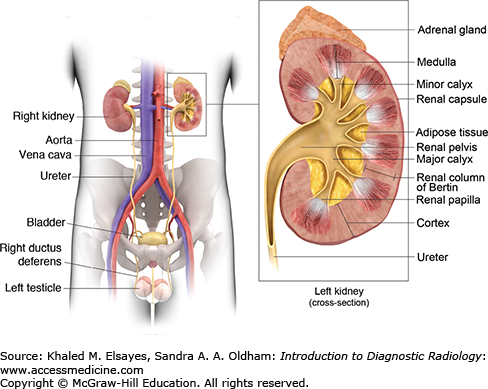
The kidneys are located in the retroperitoneum on either side of the spine. The left kidney is slightly higher than the right one and it typically extends from the level of T12 to the level of L3 vertebral body. The adrenal gland is located on top of each kidney. Both the kidney and adrenal gland are surrounded from the inside to the outside by the perinephric fat, the renal fascia (Gerota fascia), and the paranephric fat.
The kidney is a bean-shaped structure harboring the hilum on the medial side. Through the hilum, the renal artery enters the kidney posterior to the renal vein and anterior to the renal pelvis.
The internal structure of the kidney, the renal parenchyma, is composed of a superficial cortex and a deep medulla. The medullary pyramids are triangular with the base directed outward toward the cortex and the apex directed inward toward the renal pelvis. The renal columns of Bertin are an invagination of the renal cortex between the medullary pyramids. The tip of the pyramid extends to the collecting system and is called the renal papilla. The renal papilla is surrounded by the minor calyx, which collects the urine coming from the papilla of the renal pyramids. Two or three minor calyces unite to form the major calyx. Major calyces in turn unite forming the renal pelvis, which gives forth the ureter (Fig. 6.1).
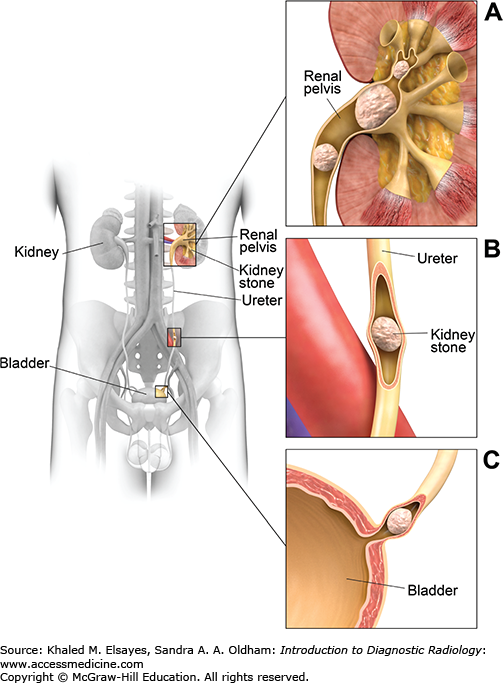
The ureter is a muscular tube, 25 to 30 cm in length. It descends in the retroperitoneum downward and medially in front of the psoas muscle, opposite the tips of the lumbar transverse processes up to the pelvic brim, where it crosses over the end of the common iliac artery or the beginning of the external iliac artery. The ureter then runs along the lateral pelvic wall until it reaches the level of the ischial spine, where it courses anterior and medially to enter the urinary bladder at its posterior inferior surface at the vesicoureteral junction. The ureter has three areas of relative narrowing in its course. These are common sites for stone impaction: at the pelviureteric junction, where it crosses the pelvic brim and at the ureterovesical junction.
The urinary bladder lies in the pelvis with the peritoneum covering only its superior surface. On its posterior surface, the ureters pass through the bladder wall for 2 cm in an oblique course before they open into the urinary bladder cavity by slitlike apertures. The two ureteric orifices are joined with the interureteric ridge. The openings of the ureters together with the opening of the urethra define the boundaries of the bladder trigone (Fig. 6.2).
CASE 1: RENAL STONE
A 40-year-old woman presents to the emergency department with a sudden onset of colicky left flank pain radiating to the upper thigh and hematuria that started 12 hours previously.
Ureteral stone
Noncontrast computed tomography (CT) is the most accurate technique, according to American College of Radiology appropriateness criteria. Advantages of CT:
High sensitivity (95%-98%) and specificity (96%-100%)
Short examination time
No need for contrast
No patient preparation and not operator-dependent like sonography
Ability to obtain coronal reformatted images
Can detect extraurinary causes of flank pain such as appendicitis, diverticulitis, and gynecological problems
Disadvantages of CT: Radiation exposure. A low-dose protocol has been developed for young patients, pregnant patients in the second and third trimester, and patients who require repeated imaging.
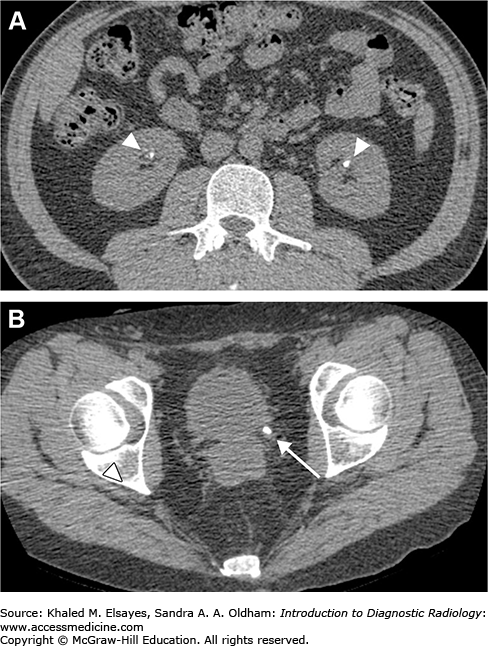
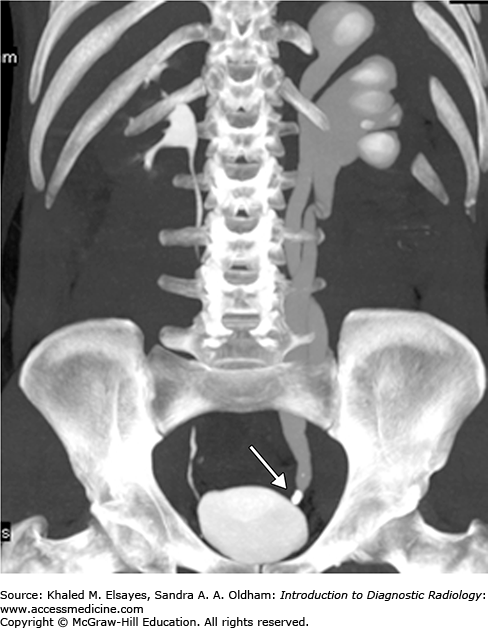
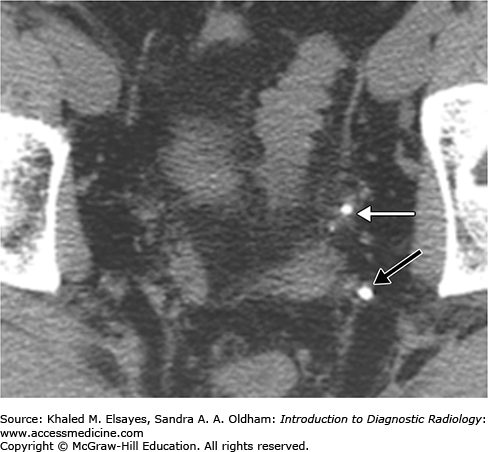
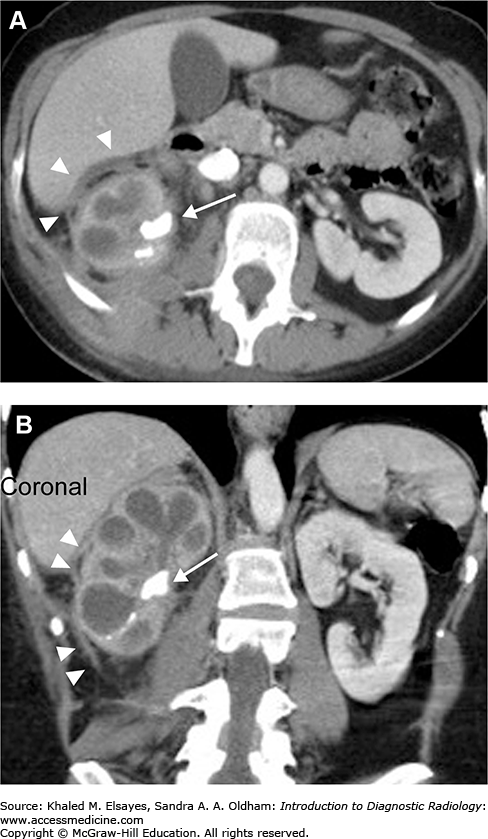
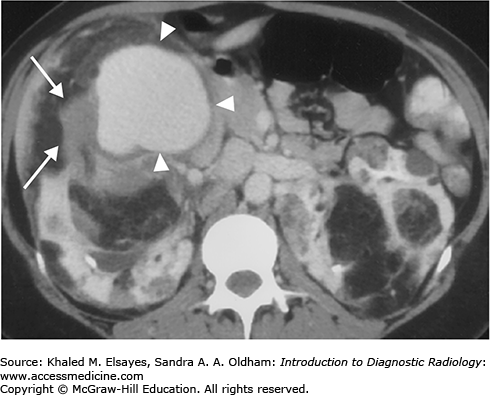
Direct detection of the stone as a rounded or oval CT-dense structure in the urinary tract (Fig. C1.1) (except stones complicated by indinavir, a protease inhibitor used in HIV-positive patients, which is CT radiolucent). CT can accurately determine the stones’ size, location (Fig. C1.2), and number, which are the most important factors affecting clinical decision.
If obstructive, the following findings can be seen (Fig. C1.3):
Dilation of the proximal ureter and renal pelvis (hydroureter and hydronephrosis).
Unilateral renal enlargement.
Stranding of the perinephric and periureteral fat.
In chronic obstruction, some patients may develop hydronephrosis, with thinning of the renal parenchyma in chronic cases.
If seen on contrast-enhanced CT, the affected kidney can have decreased or delayed concentration and excretion of contrast material.
Dual-energy CT is an evolving technique that can be used to detect the chemical composition of the stone, which may affect the choice of therapy, by providing information about how the stone behaves in different energies. For example, to differentiate calcium-containing stone from uric acid-containing stone, the postprocessing algorithm can provide color-coded images in which the voxels containing calcium are coded in blue and those containing urate are coded in red. Dual-energy CT can also be used to detect urinary stones in the contrast-filled collecting system through its ability to generate virtual unenhanced images from the original contrast-filled images.
Ureteral stones should be differentiated from other causes of flank pain in women, including appendicitis, diverticulitis, ruptured ectopic pregnancy, torsion of ovarian cyst, and enterocolitis.
Phlebolith, which is a calcification within a vein. Differentiated by:
Anatomical location outside the urinary tract.
Absence of secondary signs of obstruction.
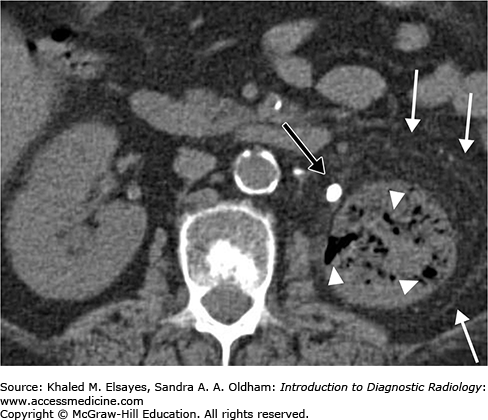
Tissue rim sign: This sign is specific for stone. It refers to the presence of a halo of soft tissue surrounding the stone representing the mural edema of the ureter (Fig. C1.4).
Comet tail sign: Can be seen with phlebolith. It refers to the presence of eccentric soft tissue extending from the phlebolith representing the collapsed vein.
Central lucency present in phlebolith and absent in stones.
From noncalcular causes of hydroureter and hydronephrosis such as urinary tract malignancies and external compression.
CASE 2: ACUTE PYELONEPHRITIS
A 35-year-old woman presents at the emergency department with increased burning micturition, right flank pain, costovertebral angle tenderness, a high-grade fever (102°F), and rigors.
Acute pyelonephritis
CT of the abdomen and pelvis, with and without contrast.
Usually, patients with acute pyelonephritis are diagnosed on a clinical basis, without imaging. However, imaging is indicated if there is no response to treatment within the first 72 hours; the patient is at high risk of developing serious complications, such as with diabetic and immunocompromised patients; when the patient has congenital or anatomic genitourinary anomalies; or to assess the severity of complications.
Women are affected more often than men. The source of infection is usually from the lower urinary tract, with E coli being the most common causative organism.
The goal is to confirm the diagnosis and detect complications.
The CT protocol includes unenhanced followed by contrast-enhanced images taken 50 to 90 seconds after contrast injection to image the kidney in the nephrographic phase. A delayed phase may also be needed when obstruction of the urinary tract is suspected.
Unenhanced images may reveal mild renal enlargement, stranding of the perirenal fat planes, and thickened Gerota’s fascia. Mild cases may have no abnormal findings, however.
When focal, acute pyelonephritis typically includes wedge-shaped areas of low attenuation or hypoenhancement that extend from the renal papilla to the cortical surface. The diffuse form may demonstrate a striated nephrogram (Fig. C2.1).
Abscesses can complicate acute pyelonephritis, especially in diabetic patients. An abscess is clinically suspected when the appropriate treatment does not resolve symptoms. CT usually demonstrates parenchymal or extraparenchymal rounded or oval cystic lesions with marginal enhancement. The inflammatory process can spread to adjacent structures, such as the psoas muscle.
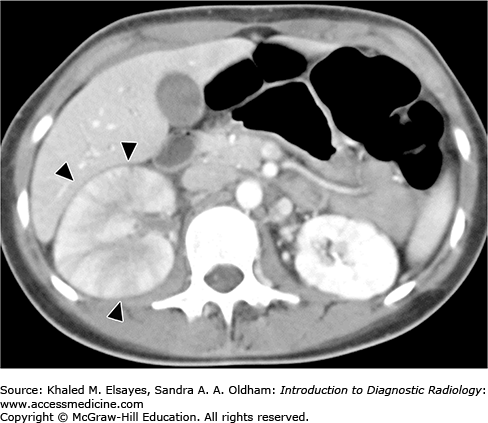
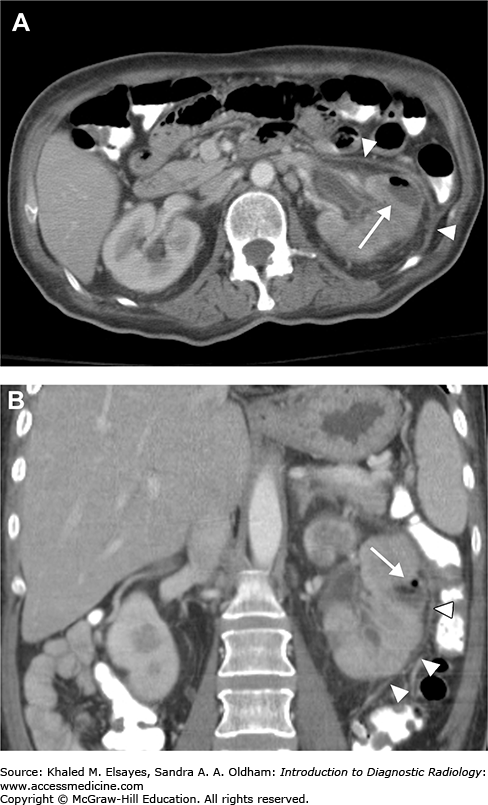
Emphysematous pyelonephritis: This severe necrotizing form of pyelonephritis occurs in diabetics, immunocompromised patients, and patients with urinary tract obstruction. The most common organism is Escherichia coli; less common organisms include Klebsiella, Pseudomonas, and Candida. The characteristic finding is the presence of gas foci in renal parenchyma or surrounding the kidney (Fig. C2.2). Emphysematous pyelonephritis is a medical emergency, and prompt treatment with IV antibiotic is needed as early as possible and relief of urinary obstruction. In severe cases, nephrectomy is performed. If not properly treated, rapid progression with fulminant sepsis can occur.
Emphysematous pyelitis: In this less aggressive type of emphysematous pyelonephritis, gas is not involving the renal parenchyma and is localized within calyceal system and the ureter.
Fungal pyelonephritis: Fungal pyelonephritis is less common than is bacterial. Candida albicans is the most common pathogen. CT may reveal fungus balls in the collecting system and multiple hypodense lesions in the renal parenchyma, which represent microabscesses.
Fig. C2.2
Noncontrast axial CT of the abdomen in a patient with emphysematous pyelonephritis reveals multiple foci of gas density in the left kidney (arrowheads) and stranding of the perinephric fat (white arrows). Note the presence of a left ureteric stone (black arrow), which may be a predisposing factor for this severe type of renal infection.
Pyelonephritis should be differentiated from other causes of flank pain in women, including appendicitis, diverticulitis, ruptured ectopic pregnancy, torsion of ovarian cyst, enterocolitis, and ureteric stone.
Pyelonephritis should be differentiated from other causes of focal hypodense parenchymal lesions, including renal infarcts and renal masses, such as renal lymphoma and renal cell carcinoma (RCC).
CASE 3: XANTHOGRANULOMATOUS PYELONEPHRITIS
A 40-year-old diabetic woman with a history of recurrent urinary tract infection presents with right flank pain, hematuria, a low-grade fever, and malaise.
Pyelonephritis
CT of the abdomen and pelvis with IV contrast
The classic imaging findings of xanthogranulomatous pyelonephritis are renal enlargement, staghorn stone, and partial or complete renal function compromise. There is destruction and replacement of the renal parenchyma, with multiple low-attenuating areas that represent xanthomatous tissue and dilated calyces filled with debris (Fig. C3.1).
Fig. C3.1
Axial (A) and coronal (B) postcontrast CT scan of the abdomen in a patient with xanthogranulomatous pyelonephritis reveals enlargement of the right kidney with a large staghorn stone (arrow) in the renal pelvis causing hydronephrosis with multiple low-attenuation areas. There is thickening of the Gerota’s fascia (arrowheads), which is seen in all types of inflammatory conditions of the kidney.
Xanthogranulomatous pyelonephritis is an uncommon atypical form of chronic infection due to an abnormal immune reaction to bacterial infection. E coli and Proteus mirabilis are the most common pathogens. Risk factors include chronic urinary tract obstruction and diabetes mellitus.
Patients are usually middle-aged women with a fever, malaise, weight loss, and flank pain. An examination may reveal a renal mass, and urinalysis reveals hematuria and pyuria.
The inflammatory process can be localized to the kidney (stage 1), extend to the perirenal space (stage 2), or spread to the pararenal spaces (stage 3). The disease process can be focal; however, most cases have diffuse involvement of the whole kidney.
Nephrectomy is the definitive treatment. A partial nephrectomy is indicated for the focal form, whereas total nephrectomy is indicated for the diffuse form.
The clinical differential diagnosis includes renal inflammatory lesions such as pyelonephritis and renal abscess.
The disease can simulate a renal neoplasm, especially when focal. These neoplasms include renal cell carcinoma, transitional cell carcinoma, renal lymphoma, and metastases. Diffuse form can be simulated by obstructive hydronephrosis caused by large calculus.
CASE 4: RENAL ABSCESS
A 44-year-old woman with a history of diabetes presents to the emergency room with a 1-week history of high fever, chills, left flank pain, and dysuria. The patient has a history of recurrent urinary tract infections.
Renal infection/inflammatory condition
CT of the abdomen and pelvis, with and without contrast
Intravenous drug abusers and patients with diabetes mellitus, AIDS, and urinary tract anomalies and obstruction are at increased risk for developing renal abscesses. The infection can reach the kidney, most commonly through an ascending infection from a lower urinary tract infection; however, hematogenous spread can also occur, as in patients who are intravenous drug abusers.
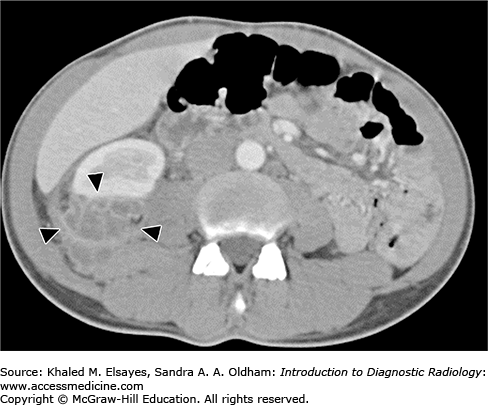
On unenhanced CT, the kidney is usually enlarged, showing a single or, less commonly, multiple ovoid, well-defined parenchymal or extraparenchymal (perinephric) hypoattenuating lesions with thick walls. Occasionally, gas density can be seen in the lesion. Gerota’s fascia is thickened, with stranding of the perinephric fat (Fig. C4.1). The perinephric abscess is usually confined by the Gerota’s fascia although it can extend to adjacent structure (Fig. C4.2). Intravenous contrast is important to differentiate the abscess from a tumor. A renal abscess has rim enhancement of the abscess wall, with no internal enhancement, whereas renal cell carcinoma (RCC) has enhancement of the whole mass, which may acquire a heterogeneous pattern because of areas of hemorrhage and necrosis. However, the most important key of differentiation remains the clinical history and urinalysis, as RCC can be cystic and demonstrate enhancement of its outer thickened wall, which may simulate the appearance of abscess.
Acute pyelonephritis: Renal abscesses usually develop as a complication of acute pyelonephritis. Imaging is needed to diagnose the abscess after treatment failure.
Other causes of intra-abdominal abscess, such as pancreatic and pericolic abscesses.
Cystic RCC, which usually presents as a solitary hypervascular or complex cystic mass with thickened. septations or solid components with no inflammatory symptoms.
Complicated renal cyst, such as a hemorrhagic or infected cyst, presents with no perinephric inflammatory reaction and is hyperattenuating on unenhanced CT.
Renal lymphoma, which more commonly presents with multiple lesions and occasionally as diffuse infiltration. Involvement of the perinephric region may be seen.
Metastases, for which a history of primary tumor is essential. The lesions are usually multiple and show contrast enhancement.
CASE 5: NEPHROCALCINOSIS
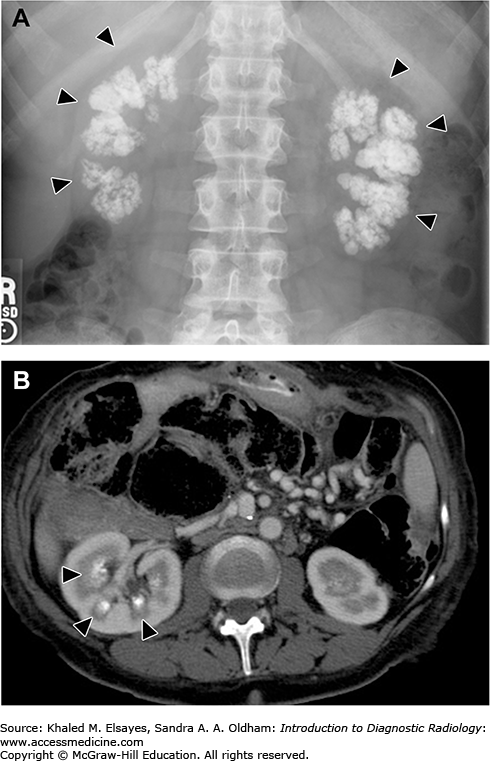
A 34-year-old man with back pain underwent plain radiography of the lumbar spine, which revealed an osteoporotic spine and calcification in the renal pyramids. A laboratory investigation reveals increased PTH and serum calcium.
Hyperparathyroidism with nephrocalcinosis
Unenhanced CT scan of the abdomen and pelvis is the modality of choice. It has a higher sensitivity than plain radiography.
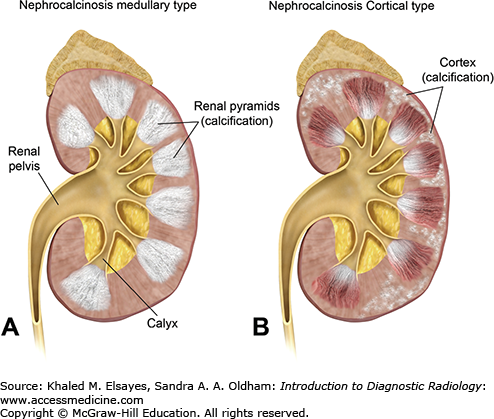
Nephrocalcinosis is a condition in which there is a large amount of calcium deposition in the renal parenchyma. It can be classified, according to the site of calcium deposition, as medullary or cortical (Fig. C5.1). Medullary nephrocalcinosis is the most common form and can be seen in patients with hyperparathyroidism, type 1 renal tubular acidosis, medullary sponge kidney, and sarcoidosis. Cortical nephrocalcinosis usually develops secondary to chronic glomerulonephritis, renal cortical necrosis, Alport syndrome, and chronic rejection of transplanted kidneys. Rarely, nephrocalcinosis involves both the renal medulla and cortex.
CT reveals calcification in the renal parenchyma. The location and pattern of calcification differs in each type. In cortical nephrocalcinosis, the calcification is seen in the periphery of the renal parenchyma, either as a peripheral rim or tram-track appearance. In medullary nephrocalcinosis, the calcification is seen only involving the renal pyramids, with unaffected areas in between representing the column of the renal cortex (Fig. C5.2).
Fig. C5.2
(A) Plain x-ray of medullary nephrocalcinosis reveals a bilateral coarse, confluent, radiopaque calcification, which is seen projecting over the renal shadows (arrowheads). (B) Axial CT scan of the abdomen of another patient reveals calcification in the medullary pyramids of the right kidney (arrowheads).
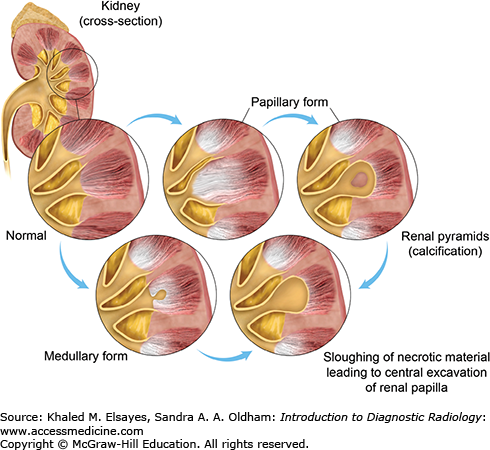
Papillary necrosis: The clinical scenario and imaging play important roles in differentiating it from medullary nephrocalcinosis. Papillary necrosis can be caused by analgesic nephropathy, diabetes mellitus, alcoholism, and sickle-cell anemia. There is damage of the renal papilla, with resultant necrosis, cavitation, and calcium deposition. Calcium deposition can occur in a nonsloughed necrotic papilla, in a completely detached papilla which remain in the calyx or at the periphery of a necrotic sloughed papilla. Unenhanced CT reveals a triangular or ring-shaped calcification in the sloughed papilla (Fig. C5.3).
Renal tuberculosis: In patients with pulmonary tuberculosis, the organism can reach the kidneys hematogenously.
The disease is unilateral, with focal amorphous or granular calcification. The kidney is atrophic, with stricture of the pelvicalyceal system.
Renal infection with Pneumocystis jiroveci: This condition occurs only in AIDS patients with low CD4 counts. It can reach the kidneys through hematogenous or lymphatic routes. The calcification is usually punctate and involves the cortex and medulla.
Fig. C5.3
Types of calyceal deformities in papillary necrosis. In the papillary form, the necrosis starts at the fornices and the necrotic papilla may remain in the calyx giving a signet ring appearance. In the medullary form, the necrosis starts in the center of the papilla and sloughing of the dead tissue leads to a blunted calyx. Note the presence of calcification in necrotic papilla, which is common in patient with analgesic nephropathy.
CASE 6: ACQUIRED CYSTIC DISEASE OF UREMIA
A 48-year-old man presents with a long history of chronic renal failure; he is on dialysis. An abdominal sonographic evaluation for flank pain reveals multiple bilateral renal cysts.
Acquired renal cystic disease
Abdominal sonography can be used as a screening examination to establish the diagnosis; however, contrast-enhanced CT is required to detect complications, especially renal cell carcinoma (RCC), which develops in about 7% of these patients.
Acquired renal cystic disease refers to the development of three or more renal cysts in each kidney in patients with end-stage renal disease. The condition is more common in men and patients undergoing dialysis, irrespective of the method. Its incidence increases with dialysis duration.
Sonography reveals multiple bilateral renal cysts (at least 3 on each side) that appear as anechoic structures with posterior acoustic enhancement. Imaging features of end-stage renal disease are also seen, including bilaterally small kidneys, increased parenchymal echogenicity, and loss of corticomedullary differentiation.
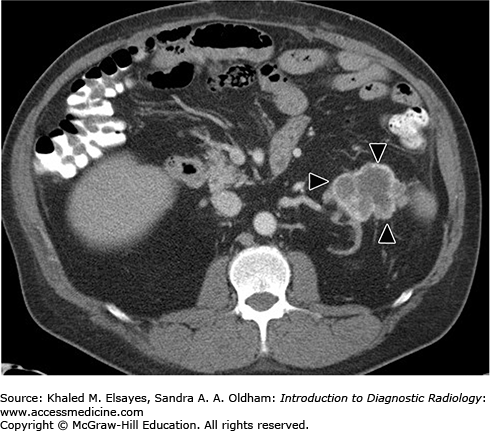
A CT scan reveals atrophic kidneys with multiple parenchymal cysts. Sometimes hemorrhage inside a cyst or calcification in a cyst wall can be seen. CT can detect RCC, which requires intravenous contrast administration and appears as a focal, solid hypervascular lesion (Fig. C6.1).
The patient is usually asymptomatic and diagnosis is often made in the workup of other complaints
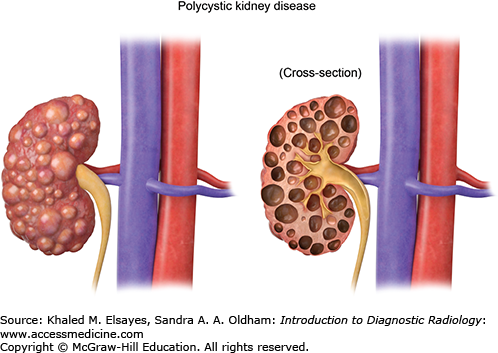
It is characterized by autosomal dominant inheritance and massive enlargement of both kidneys, with bilateral, innumerable noncommunicating cysts. The cysts usually begin to appear by age 30 and increase in size over time. They diffusely involve the whole kidney, changing its normal renal contour (Figs. C6.2 and C6.3). Complicated cysts, such as hemorrhagic or infected cysts, can be identified on CT by increased attenuation or the presence of gas, respectively. Cysts can also involve the liver, spleen, pancreas, and ovaries. There is no association between adult polycystic kidney disease and RCC. Adult polycystic kidney disease can however lead to end-stage renal disease.
It is an autosomal dominant disorder that affects multiple systems. The condition is due to a mutation of the VHL tumor suppressor gene located on chromosome 3. Patients with this mutation can develop hemangioblastomas in the retina, cerebellum, and spine and pancreatic cysts, pancreatic cystic neoplasms, pheochromocytoma, and epididymal cystadenomas. The kidneys are of normal size and function, with multiple bilateral, variable-sized cysts. Complex cysts or cysts with mural nodules can be seen and can suggest malignancy. RCC can develop at a younger age and, along with neurological complications, is a cause of death in these patients.
It is an autosomal dominant disorder characterized by bilateral renal cysts, multiple renal angiomyolipomas (see case 10), and CNS lesions, including cerebral periventricular calcifications, cortical tubers, and subependymal nodules. Patients may present with seizures due to CNS lesions.
See case 7.
CASE 7: RENAL CYSTS
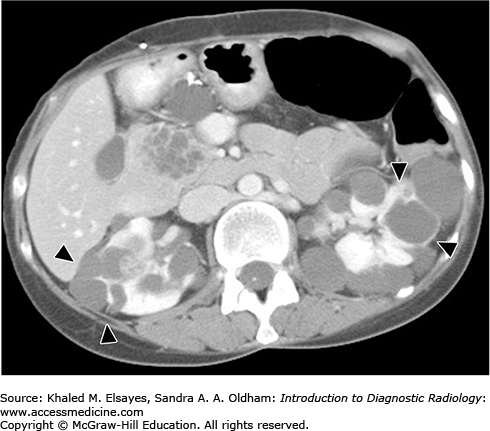
A 38-year-old man presents with vague left-side abdominal pain; abdominal sonogram was normal, apart from large left renal cysts.
Simple renal cyst
CT of the abdomen, with and without intravenous contrast, is the modality of choice for assessing indeterminate renal lesions. Sonography can be used to distinguish a simple benign cyst that needs no further workup from a complicated cyst that requires further evaluation by CT. However, multiple factors can affect proper sonographic evaluation, such as the patient’s body habitus, small lesions, and cyst wall calcification.
On sonography, simple cysts appear as well-defined, thin-walled anechoic lesions with posterior acoustic enhancement and no internal echoes, septations, or solid nodules. On CT, simple cysts appear as fluid density lesions with no wall enhancement after intravenous contrast.
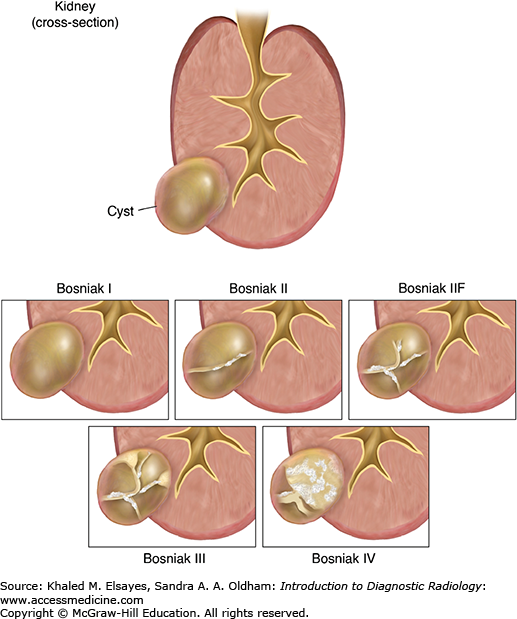
Dr. Morton Bosniak created the Bosniak classification system on the basis of CT imaging findings to categorize cystic renal lesions into 5 categories (Fig. C7.1). This classification helps in clinical management by classifying these lesions as surgical or nonsurgical.
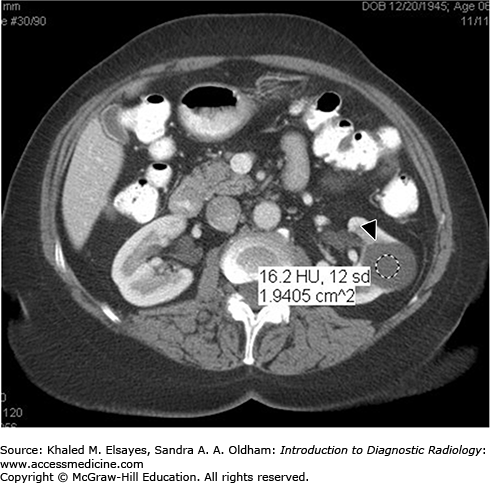
Category I:Simple uncomplicated cyst, which contains water density fluid and has a thin wall, with no septations, calcifications, solid components, or cyst wall enhancement after intravenous contrast (Fig. C7.2). Cysts in this group are benign and no follow-up is needed.
Category II:Minimally complicated cyst, including homogenous high-attenuation cysts and cysts with few thin septae, a fine rim of calcifications, or a short segment of thickened calcifications. Cysts in this group are benign and no follow-up is needed.
Category IIF: Lesions in this category require follow-up and include cysts with increased numbers of septae, minimal mural or septal thickening, or thick calcium in the wall. Follow-up is done by CT or MRI after 6 months, then every year. If the lesion remains stable for 5 years, it is considered benign.
Category III: More complicated cyst that contains a thickened, enhancing wall or internal septations. Lesions in this group are indeterminate and require surgical excision (partial nephrectomy).
Category IV: Clearly malignant cystic lesion. In addition to the criteria in group III, the cyst contains solid and soft tissue components. Lesions in this group are managed by nephrectomy.
Symptomatic renal cysts must be differentiated from other causes of abdominal pain, such as cholecystitis.
Cystic RCC
Renal abscess
Cystic metastases
CASE 8: RENAL TRANSPLANT DYSFUNCTION
A 51-year-old man underwent a live donor renal transplant and presents with hypertension 2 weeks later. Laboratory studies revealed a high creatinine level.
Complicated dysfunctioning transplant due to rejection or vascular compromise
Color and power Doppler sonography
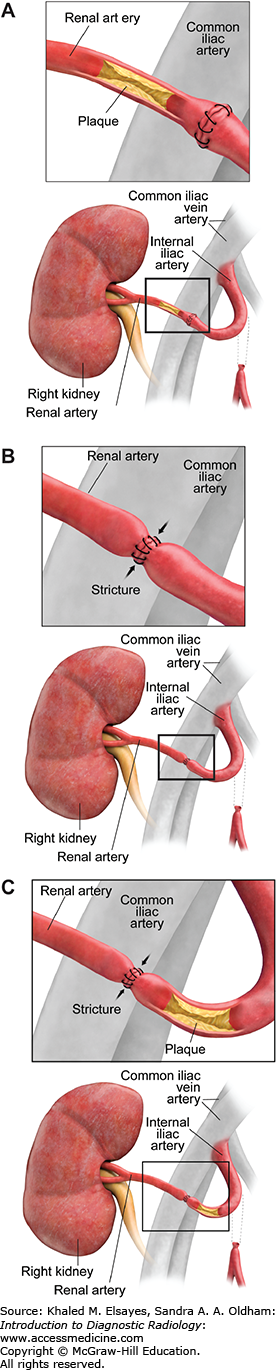
Renal artery stenosis is the most common vascular complication after renal transplantation. The underlying mechanism includes atherosclerosis of the donor or recipient arteries and iatrogenic causes, such as faulty suture technique, surgical injury to the vessel, or vascular kink. The site of stenosis can be in the recipient iliac artery, at the anastomotic site, or in the donor renal artery (Fig. C8.1).
The condition can be suspected clinically if there is severe hypertension that is refractory to treatment or unexplained impaired renal function after renal transplantation.
Sonography is used to visualize blood flow, measure its speed, and characterize its waveform. Normally, the peak systolic velocity is less than 150 cm/s at the main renal artery. Spectral analysis of the normal Doppler waveform demonstrates rapid systolic upstroke peak and low-resistance continuous forward flow throughout the cardiac cycle.
In case of renal artery stenosis, the site of stenosis will demonstrate increased peak systolic velocity of the flowing blood with a focal area of color aliasing, which can be simply defined as color mixture and spill outside the vessel lumen at the narrowed area (Fig. C8.2A). The velocity gradient between the stenotic and prestenotic segments is increased. Poststenotic turbulent flow is also present. Intrarenal waveforms will also be abnormal, demonstrating presence of a small amplitude waveform with a prolonged systolic rise (slow upstroke), known as parvus tardus waveform (Fig. C8.2B).
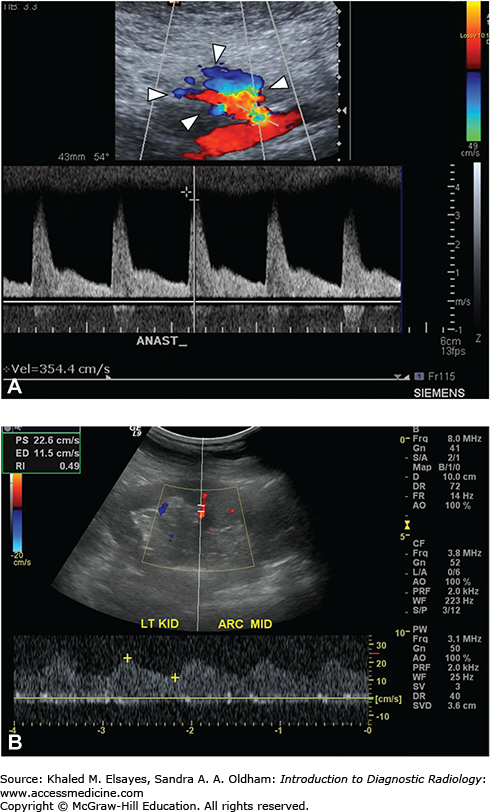
Fig. C8.2
A 24-year-old male presented with hypertension following renal transplant. (A) Spectral Doppler sonogram of transplant renal artery at site of anastomosis demonstrates focal area of color aliasing (arrowheads) with significantly increased systolic upstroke; elevated PSV (peak systolic velocity of 354 cm/s). (B) Longitudinal sonogram of renal transplant shows parvus tardus waveforms in segmental artery with mildly decreased color flow in kidney.
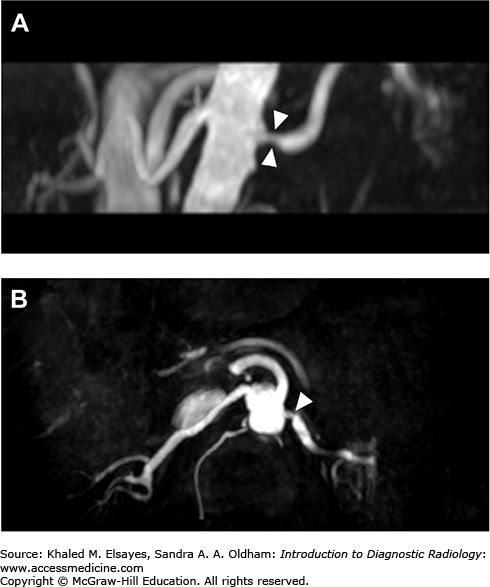
The renal artery can also be evaluated using MR angiography (Fig. C8.3). The artery can be visualized without contrast administration, but artifacts from surgical clips may affect image quality.
Other causes of graft failure include:
Rejection
Urinary obstruction
Acute tubular necrosis
Large hematoma
Other vascular complications of renal transplantation include:
Intrarenal arteriovenous fistula
Renal artery thrombosis
Renal graft torsion
Renal vein thrombosis
Renal infarction
CASE 9: RENAL CELL CARCINOMA
A 55-year-old man presents with fatigue, painless hematuria, and unexplained weight loss.
Renal cell carcinoma (RCC)
Multiphasic CT scan of the abdomen and pelvis is the modality of choice. Imaging is important for accurate staging, which affects treatment options and surgical planning (Table C9.1). The scanning protocol consists of unenhanced CT, followed by intravenous contrast administration and image acquisition in the corticomedullary and nephrographic phases. The corticomedullary phase is performed after 60 seconds of contrast injection and is important for visualizing the renal vein and detecting distant organ metastases, whereas the nephrographic phase is performed after 80 seconds of contrast injection and is the most sensitive for tumor detection. The excretory phase is optional and performed after 2 to 5 minutes.
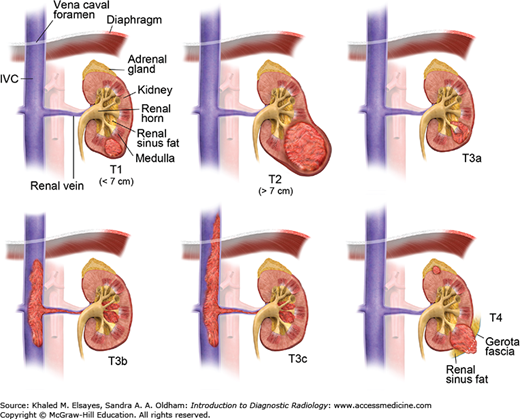
The American Joint Committee on Cancer (AJCC) Staging of Renal Cell Cancer
| Stage | Description |
|---|---|
| T0 | No evidence of primary tumor |
| T1a | Tumor is limited to the kidney and is <4 cm in greatest diameter |
| T1b | Tumor is limited to the kidney and is 4-7 cm in greatest diameter |
| T2a | Tumor is limited to the kidney and is 7-10 cm in greatest diameter |
| T2b | Tumor is limited to the kidney and is >10 cm in greatest diameter |
| T3a | Tumor invades: renal vein or its segmental branches. Perirenal and/or renal sinus fat (no extension beyond Gerota’s fascia or to the ipsilateral adrenal gland). |
| T3b | Tumor invade the IVC below the level of the diaphragm |
| T3c | Tumor invade the IVC above the level of the diaphragm |
| T4 | Invasion beyond Gerota’s fascia or to the ipsilateral adrenal gland |
| N0 | No nodal involvement |
| N1 | Regional lymph node involvement |
| M0 | No distant metastases |
| M1 | Distant metastases |
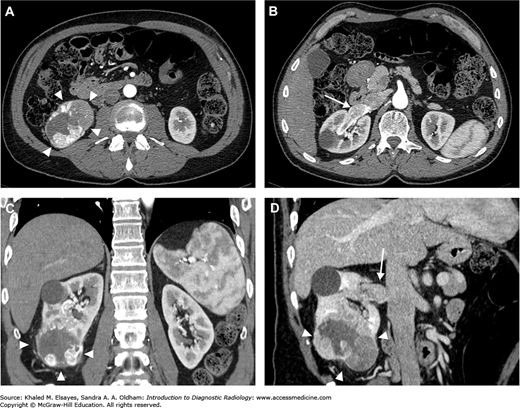
Unenhanced CT may reveal a circumscribed, usually solid mass arising from the renal cortex. Depending on the size of the mass, it may cause distortion of the normal renal contour. The attenuation of the lesion may be heterogeneous because of the presence of intratumoral hemorrhage, necrosis, and, less commonly, calcification. After intravenous contrast, the tumor usually enhances, albeit less than the renal parenchyma (Fig. C9.1A,C). Enhancement more than 15 to 20 HU after intravenous injection of contrast is considered significant and highly raises the suspicion of RCC. The papillary type of RCC is hypovascular and can be mistaken for a cyst.
CT is important in disease staging. It can detect multifocal lesions, perinephric tumor extension, renal sinus fat invasion, and extension to the adjacent organs, including the ipsilateral adrenal gland (Fig. C9.2). Although uncommon, it is important to evaluate the contralateral kidney to exclude bilateral RCC.
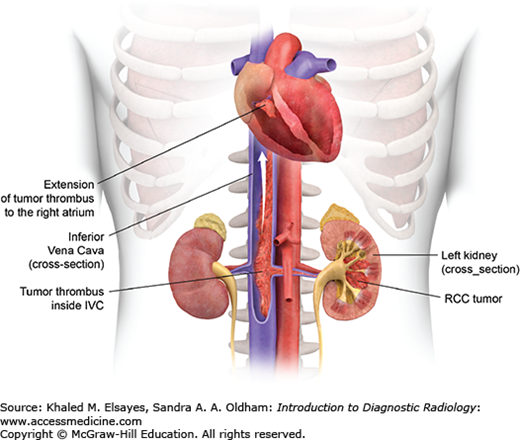
Furthermore, the tumor can grow directly into the renal vein, reaching the IVC; it may even extend to the right side of the heart (Fig. C9.3). This is best visualized in the corticomedullary phase of enhancement, which demonstrates a filling defect in the enhanced vein in direct continuity with the primary tumor (Fig. C9.1B,D).
Lymphatic spread occurs to the regional lymph nodes. Hematogenous spread can lead to the development of hypervascular metastasis, most commonly involving the lungs, liver, bones, and brain. Rare locations include the pancreas and thyroid gland.
RCC should be differentiated from other causes of gross hematuria, such as those originating from the collecting system, ureter, or a urinary bladder carcinoma.
RCC should be differentiated from other renal masses, including:
Angiomyolipoma (AML): AML are predominantly fatty lesions (see case 10).
Oncocytoma: Oncocytoma is a benign epithelial tumor arising from the collecting ducts. this lesion is usually homogenous and solid, yet pathological diagnosis is usually needed.
Lymphoma: Lesions are usually multiple and bilateral.
Hemorrhagic renal cyst: This can be differentiated from tumor by lack of significant enhancement on postcontrast series.
CASE 10: RENAL ANGIOMYOLIPOMA
A 43-year-old woman presents with vague abdominal pain. Sonography reveals an incidental hyperechoic mass lesion within the left kidney.
Renal angiomyolipoma
CT of the abdomen, with and without contrast
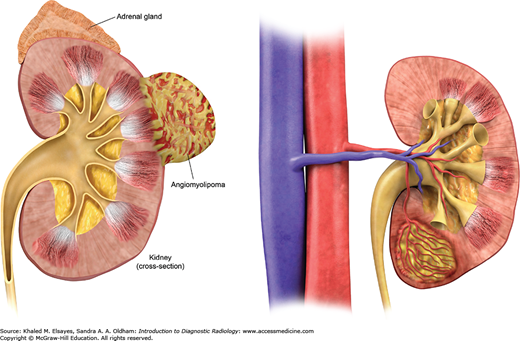
Angiomyolipoma (AML) is the most common benign tumor of the kidney. These tumors are composed of macroscopic fat, blood vessels, and smooth muscles in different proportions (Fig. C10.1). It ranges in size from a few millimeters to several centimeters or even larger. Angiomyolipoma can be single or multiple. Multiple angiomyolipomas can be seen in patients with tuberous sclerosis (Fig. C10.2).
On CT, AML appears as a well-defined cortical lesion. The presence of intralesional fat density (low-density areas of -30 to -100 HU) is an important clue for diagnosis. However, when the lesion is small, it may be difficult to differentiate from small cyst or other lesions.
On MRI, AML will demonstrate bright signal on non-fat-suppressed T1-weighted images in both in and opposed phase and will lose signal only in fat-suppressed sequences because the fat in AML is macroscopic (Fig. C10.3).
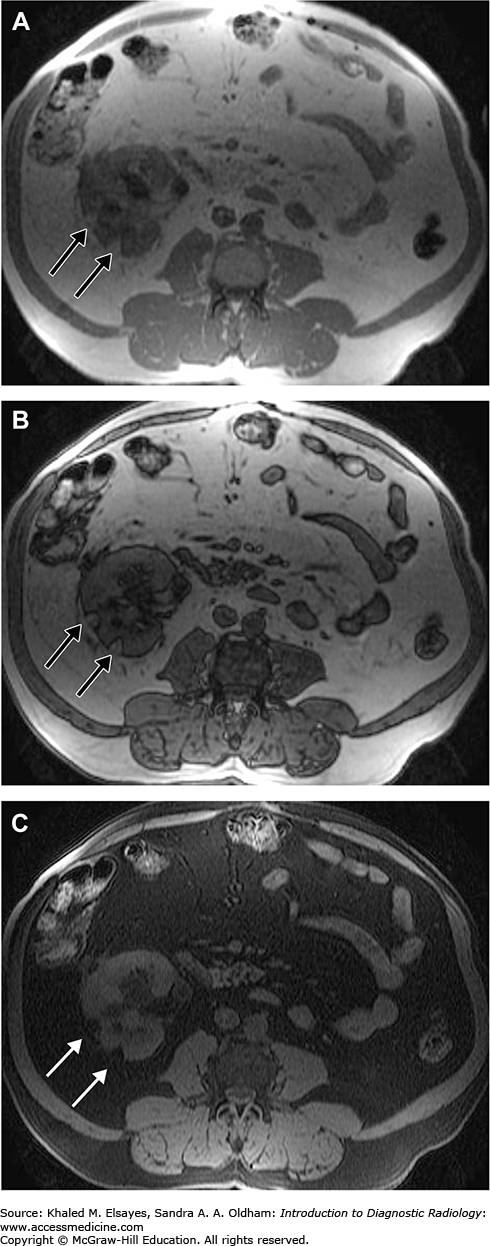
Fig. C10.3
Axial T1WI in-phase (A), out-of-phase (B) and fat suppression of the abdomen in a patient with angiomyolipomas reveals two well-defined left renal fat-containing mass lesions with a bright signal on T1WI in-phase and out-phase (black arrow, A and B). Note the loss of signal of the lesion in the fat suppressed (white arrow, C) but not in the out of phase image denoting the presence of macroscopic fat within.
Angiomyolipomas may be fat-poor, especially in the setting of tuberous sclerosis, as up to one-third do not demonstrate macroscopic fat on CT. Calcification is rare.
AML can be complicated with rupture and hemorrhage (Fig. C10.4). This can be seen in large tumors (diameter greater than 4 cm). Patient will present with acute abdominal pain and CT will demonstrate perinephric hematoma. Control of hemorrhage can be done by embolization of the bleeding vessel followed by partial or complete nephrectomy.
Spontaneous renal bleeding secondary to an AML usually occurs in tumors larger than 4 cm.
AML is usually asymptomatic. However, in cases of spontaneous rupture it will present with acute abdomen, and workup should be performed to differentiate it from other causes of acute abdomen.
There is essentially no differential diagnosis for typical AML (as the dominant presence of macroscopic fat is virtually pathognomonic for angiomyolipoma). In atypical AML, especially fat-poor, other lesions to consider include RCC, retroperitoneal sarcoma invading the kidney, oncocytoma, Wilms’ tumor, metastases, and lymphoma.
Retroperitoneal liposarcoma invading the kidney: The center of the lesion is outside the kidney and the kidney appears compressed by the lesion.
RCC: The absence of fat and presence of calcification within the lesion make AML less likely and raise suspicion of RCC.
Oncocytoma: It is a benign renal tumor and may contain fat. The lesion is usually solid, and demonstrate homogenous enhancement.
Wilms’ tumors: It may contain fat. However, this tumor occurs in the pediatric age group.
CASE 11: SEPSIS-INDUCED ADRENAL HEMORRHAGE
A 10-year-old boy with a recent history of meningitis presents with fatigue, anorexia, nausea, and vomiting.
Adrenal hemorrhage caused by sepsis, especially meningococcemia (also known as Waterhouse-Friderichsen syndrome)
CT scan examination of the abdomen (without and with intravenous contrast)
Sonography: The pattern of echogenicity of an adrenal hematoma depends on its age.
An early-stage hematoma appears solid, with diffuse or inhomogeneous echogenicity. As liquefaction occurs, the mass demonstrates mixed echogenicity with a central hypoechoic region and eventually becomes completely anechoic and cystlike.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree