15 Graft surveillance and preoperative vein mapping for bypass surgery
INTRODUCTION
Patients with significant lower-limb ischemia or threatened limb loss usually require arterial bypass surgery if no other option is available to improve blood flow in the leg. Vascular surgeons are able to perform an extensive range of arterial bypass procedures to restore circulation to the extremities. Bypass grafts can be made of synthetic materials, such as polytetrafluoroethylene (PTFE), or constructed from native vein, which can be assessed and marked preoperatively as described at the end of this chapter. Failure of a bypass graft due to the development of a graft stenosis is a serious complication that can result in amputation if it is not possible to unblock the graft. It is therefore common practice for vascular laboratories to perform regular graft surveillance scans to detect the development of graft defects. The majority of surveillance scans are performed for native vein bypass grafts below the groin (infrainguinal grafts). The surveillance of synthetic grafts is equivocal and benefits are less clear-cut (Lundell et al. 1995). Ultrasound can also be used to image areas of potential infection following graft surgery, to see if the region of infection is in contact with the graft. The emphasis of this chapter will be on infrainguinal vein graft surveillance.
ANATOMY
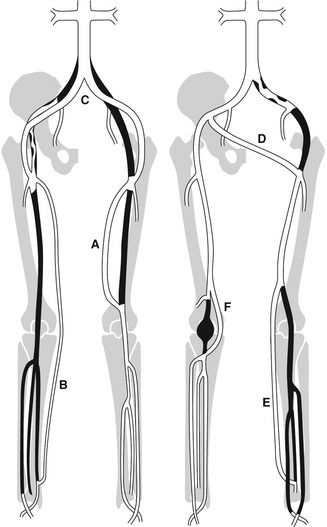
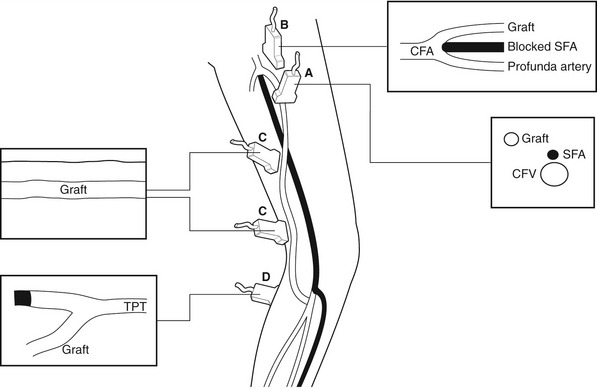
The routes of grafts vary considerably and depend on the level and extent of the native arterial disease that has been bypassed. Synthetic grafts are mainly used to bypass inflow disease (aortoiliac segment), whereas vein grafts are frequently used for distal procedures below the inguinal ligament. The different types of graft frequently encountered in the graft surveillance clinic are shown in Figure 15.1.
Vein grafts
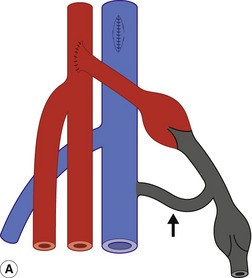
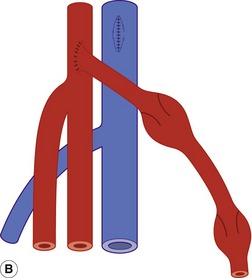
Whenever possible, native vein is used for femoral distal bypass surgery, as it offers good long-term patency rates and there is less risk associated with infection compared to synthetic grafts. The great saphenous vein is the vein of choice for infrainguinal bypass surgery, although an arm vein or the small saphenous vein can also be used if the great saphenous vein is unsuitable in part or all of its length. Vein grafts composed of more than one segment of vein are known as composite vein grafts. Femoral distal bypass surgery is performed using three common types of surgical procedure (Fig. 15.2).
In the third type of procedure, the great saphenous vein is completely removed and turned through 180° so that the distal end of the vein will form the proximal anastomosis. This is called a reversed vein graft. One particular advantage of this technique is that, in this orientation, the valves will not prevent blood flow toward the foot and do not need to be removed (Fig. 15.2B). Reversed vein grafts are often tunneled deep in the thigh beneath the sartorius muscle, which can make imaging difficult. As the vein is reversed, the diameter of the proximal segment of the graft is usually smaller than the distal segment. This can result in a size mismatch between the proximal inflow artery and proximal graft that is evident on the scan. When there is insufficient length of native vein available, a combination of synthetic material and vein may be used to form a composite graft (Fig. 15.3).
Synthetic grafts
Synthetic grafts are used for aortobifemoral, iliofemoral, axillofemoral, and femorofemoral cross-over grafts. Synthetic PTFE grafts are also used for femoropopliteal bypass, but the long-term patency rates are not as good as grafts constructed from native vein (Klinkert et al. 2003). Vein cuffs or collars are sometimes used to join the distal end of a synthetic femoral distal graft to the native artery. They produce a localized dilation at the anastomosis, which is thought to reduce the risk that a stenosis will occur.
PURPOSE OF GRAFT SURVEILLANCE
Vein grafts
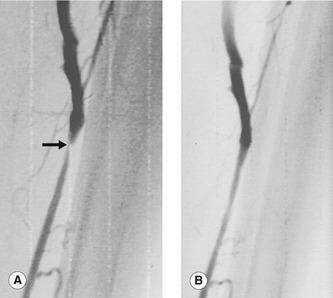
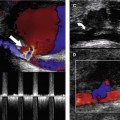
The development of an intrinsic vein graft stenosis is a major source of vein graft failure (Grigg et al. 1988; Caps et al. 1995). An angiogram demonstrating a graft stenosis is shown in Figure 15.4. Early graft failure, within the first month, is attributed to technical defects or poor patient selection. Such an example would be a patient with very poor run-off below the graft, resulting in increased resistance to flow and eventual graft thrombosis. Graft failure beyond 1 month is attributed to the development of intimal hyperplasia that can occur when there is damage to the endothelium of the vessel wall. This causes smooth-muscle proliferation into the vessel lumen and subsequent narrowing. Stenoses can occur at any point along the graft and can sometimes be extremely short, web-like lesions. Incomplete removal of valve cusps during in situ bypass surgery can also cause localized flow disturbance and narrowing. Late graft failure, beyond 12 months, can also be due to progression of atherosclerotic disease in the native inflow or outflow arteries, above and below the graft.
Patients are normally scanned at regular intervals in the first 12 months following bypass surgery. The program used by our unit is shown in Box 15.1. Some vascular units also continue to scan patients indefinitely beyond the first year at 6-month intervals to detect late graft problems. The time interval between scans is shortened to 1–2 months if a patient shows signs of developing a moderate stenosis. Patients requiring angioplasty or surgical revision of a significant graft defect recommence the surveillance program from the beginning. It can be seen that graft surveillance programs require considerable commitment from the vascular laboratory. There therefore remains considerable debate about the usefulness of such programs. The principal results of the Vein Graft Surveillance Randomised Trial (Davies et al. 2005) indicated that intensive surveillance with duplex scanning did not show any additional benefit in terms of limb salvage rates for patients undergoing bypass surgery, but it does incur extra costs. Other studies suggest that they are effective in maintaining patency rates and are less costly than surgical revision after a graft thrombosis, or rehabilitation following amputation (Lundell et al. 1995; Wixon et al. 2000). There is also evidence that an early postoperative scan, 6 weeks after surgery, can predict which lesions will require continuing duplex surveillance (Mofidi et al. 2007), enabling the effective targeting of resources to selected patients. In practice, many departments have developed local protocols that are a hybrid of published studies.
SYMPTOMS AND TREATMENT OF GRAFT STENOSIS OR FAILURE
Most patients experience no symptoms in the presence of a developing graft stenosis, and grafts may fail without any prior warning. However, symptoms that can be attributable to imminent graft failure are the sudden onset of severe claudication or a sensation of coldness involving the foot. Urgent intervention is required in this situation to prevent graft occlusion. Most graft stenoses are treated successfully by balloon angioplasty. However, recurrent stenoses sometimes require surgical revision involving local patching of a defect or partial graft replacement using a new segment of vein. Early graft occlusion can be treated by thrombolysis or graft thrombectomy. There is often an underlying cause for the occlusion that requires correcting, such as a graft stenosis, inflow stenosis, or run-off occlusion. Conversely, some grafts develop a local aneurysm that may become so large that a segment of graft has to be replaced.
PRACTICAL CONSIDERATIONS FOR SCANNING BYPASS GRAFTS
What does the clinician want to know?
• Is there a graft stenosis and where is it located?
• What is the peak systolic velocity (PSV) across the stenosis?
• What is the PSV ratio across the stenosis?
• Estimate the degree of stenosis
• Does flow appear compromised in the graft (damped flow, low-velocity flow, or very high-resistant flow)?
• Is there evidence of inflow or outflow obstruction?
• Are there any graft aneurysms? Measure their size and position
• Is there evidence of graft entrapment? State the position
• Are there abnormal fluid collections associated with the graft? State the position
• Are there any significant changes since the previous scan? If so, what are they?
No special preparation is required for the examination, and the vast majority of graft scans can be completed within half an hour. The majority of bypass scans are performed with the patient lying supine or semisupine. When scanning vein grafts, the leg should be externally rotated and the knee gently flexed and supported. It is sometimes necessary to roll the patient over to one side in order to scan the posterior lower thigh, popliteal fossa, or upper posterior calf if the graft is anastomosed to the popliteal artery. Positions for scanning the tibial arteries are discussed in Chapter 9. The scanner should be configured for a graft scan, or in the absence of a specific preset, a lower-limb arterial investigation. Adjustment of the controls is frequently necessary, especially if there is low-volume flow in the graft (see Ch. 7).
SCANNING TECHNIQUES
In situ vein graft
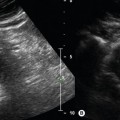
The main body of an in situ vein graft remains superficial in the leg and runs along the medial aspect of the thigh (Fig. 15.5). It is often easier to locate the graft in the upper medial thigh using a transverse imaging plane and then to follow the graft up to the proximal anastomosis (Fig. 15.5A).
The transducer is rotated into a longitudinal scan plane at the proximal anastomosis (Fig. 15.5B). Ideally, a minimum 5 cm length of the inflow artery above the graft origin should be examined to exclude any disease. For instance, damped waveforms at this level are likely to indicate significant inflow disease. The proximal anastomosis should be carefully interrogated using color flow imaging and spectral Doppler for any signs of stenosis.
The graft is then carefully followed in longitudinal section along the thigh (Fig. 15.5C) with the color pulse repetition frequency (PRF) optimized to use the full color scale to demonstrate any flow disturbances. A high-frequency transducer provides the best image of the main body of an in situ graft. Spectral Doppler measurements should be made along the length of the graft, looking for waveform changes, especially in areas demonstrating color flow changes. It is often difficult to obtain good Doppler angles when scanning superficial vein grafts, and gentle ‘heel-toeing’ of the transducer may be required. A wedge of ultrasound gel can help if a specific region needs close examination.
The distal portion of many in situ vein grafts run deep to join a native artery at the distal anastomosis (Fig. 15.5D
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree