Gynecologic Malignancies
Loren K. Mell
Josh J. Haslam
John C. Roeske
Arno J. Mundt
Interest in image-guided radiation therapy (IGRT) has increased with the growing use of conformal techniques, such as intensity-modulated radiation therapy (IMRT), particularly in women with gynecologic cancers.1 Compared to conventional techniques, IMRT is associated with prolonged treatment times and steeper dose gradients away from the planning target volume (PTV), necessitating a better understanding of how targets move and change during (intrafraction) or over (interfraction) the course of treatment. Knowledge of the systematic and random factors contributing to daily setup uncertainty is also of importance to ensure precise and accurate radiotherapy (RT) delivery. IGRT permits one to account and correct for such motions and thus deliver better treatment. The importance of IGRT will likely continue to grow as modern imaging modalities and conformal RT techniques gain wider use.
IGRT opens a door to exploring some fairly radical approaches to gynecologic RT. Unconventional fractionation, such as with simultaneous integrated boost or dose-painting IMRT techniques, has potential radiobiologic and logistical advantages over conventional approaches, but its safe delivery requires a firm understanding of the uncertainties in target localization. IGRT may similarly permit the use of hypofractionated conformal RT to boost gross disease within the pelvis. It may also allow improved normal tissue sparing and potentially alter the toxicity profile of modern combined-modality therapy. Moreover, it should also help transform brachytherapy by improving target delineation. Finally, by visualizing radiographic changes in tumor and normal tissue position and morphology, IGRT allows the possibility to systematically adapt RT plans in response to acute changes.
IMAGE-GUIDED TARGET DELINEATION
EXTERNAL-BEAM RADIOTHERAPY
The use of bony landmarks to define gynecologic RT portals has been repeatedly shown to lead to inadequate target coverage and excess normal tissue irradiation compared to three-dimensional (3D) planning.2,3 Computed tomography (CT) provides more accurate and customized target delineation and permits dose calculations based on electron density, so CT has become the standard imaging technique used today. However, compared to CT, magnetic resonance imaging (MRI) provides superior soft tissue definition, permitting better delineation of the volume and extent of pelvic tumors.4,5 MRI is also superior to physical examination for assessing uterine involvement and tumor response and better predicts local control and disease-free survival.6, 7, 8 Therefore, although presently MRI is not routinely incorporated into external-beam RT planning, in select cases, registering or fusing an MRI with the planning CT can be useful to guide target delineation.
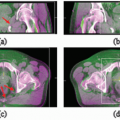
For assessing nodal metastasis, routine MRI and CT have similar accuracy.9 Several investigators have studied ultrasmall particles of iron oxide (USPIO) to improve nodal assessment with MRI. Rockall et al.10 found that USPIO-enhanced MRI increased the sensitivity of MRI, without loss of specificity, in endometrial and cervical cancers. A meta-analysis found that MRI enhanced with superparamagnetic iron oxide nanoparticles has a sensitivity of 88% and specificity of 96%, compared to 63% and 93%, respectively, for unenhanced MRI.11 Taylor et al.12 used USPIO-enhanced MRI to guide nodal delineation and found that a 7-mm margin with slight modifications was sufficient to encompass 99% of pelvic nodes (Fig. 16.1).
Numerous studies in cervical cancer have demonstrated that [18F] fluorodeoxyglucose (FDG) positron emission tomography (PET) (or PET/CT) detects cervical abnormalities and occult nodal disease with higher sensitivity than MRI or CT alone.13, 14, 15 Moreover, several reports have also shown that FDG-PET abnormalities are correlated with poorer outcomes.13,16,17 Others have found that FDG-PET improves diagnosis and staging of endometrial18 and vaginal cancer.19 However, although FDG-PET may be a useful tool to guide nodal clinical target volume (CTV) delineation for RT planning purposes, it may not have sufficient negative predictive value to obviate lymph node sampling or lymphadenectomy for patients with preoperative imaging negative for nodal metastasis.20 FDG-PET can be used to measure tumor volume and even monitor response during treatment of cervical cancer.21,22 For patients with para-aortic lymph node metastases, FDG-PET may be useful to delineate the gross disease, which, at least theoretically, can be safely boosted to 59.4 Gy.23,24
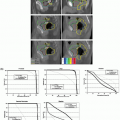
A few studies have investigated applications of advanced imaging. Dynamic contrast-enhanced MRI can identify regions of tumor hypoxia25 and 1H-magnetic resonance spectroscopy (MRS) can be useful to differentiate tumor from normal tissue (Fig. 16.2).26 Studies have also investigated PET with
alternative tracers, such as 11C-choline, 11C-methionine, and 60Cu-diacetyl-bis(N4-methylthiosemicarbazone) (60Cu-ATSM), to identify metabolic abnormalities or hypoxia.27, 28, 29, 30 Roeske et al.31 found that single-proton emission CT (SPECT) could be a useful adjunct for IMRT planning by imaging active bone marrow, which could be selectively avoided. To date, however, limited data exist to support the use of these sophisticated imaging approaches for RT planning in gynecology patients, and further study is required to define their role.
alternative tracers, such as 11C-choline, 11C-methionine, and 60Cu-diacetyl-bis(N4-methylthiosemicarbazone) (60Cu-ATSM), to identify metabolic abnormalities or hypoxia.27, 28, 29, 30 Roeske et al.31 found that single-proton emission CT (SPECT) could be a useful adjunct for IMRT planning by imaging active bone marrow, which could be selectively avoided. To date, however, limited data exist to support the use of these sophisticated imaging approaches for RT planning in gynecology patients, and further study is required to define their role.
BRACHYTHERAPY
Many investigators have described advantages of 3D imaging and volumetric dosimetry over conventional imaging modalities and standard point-dose dosimetry for gynecologic brachytherapy.32, 33, 34 CT34 and ultrasound35 are useful and relatively inexpensive imaging techniques that can both guide and improve brachytherapy. More recently, MRI and PET have received attention as means of augmenting brachytherapy planning.
Studies have found that CT can overestimate the target volume compared to MRI.36 Wachter-Gerstner et al.37 reported that MRI-guided brachytherapy planning improves estimated dose delivery to the bladder and rectal walls compared to conventional techniques. High rates of local control and low toxicity have been reported using MRI-guided approaches.38,39 Investigators at the Medical University of Vienna have developed a specialized applicator for MRI-guided brachytherapy,38 and in recent years, working groups have published recommendations for MRI-guided brachytherapy in cervical cancer.32,40,41 Although the majority of research in MRI-guided brachytherapy has focused on cervical cancer, promising results have also been reported using this technique to treat recurrent endometrial42 and inoperable uterine43 cancers.
Investigators at Washington University have conducted several studies of PET-guided intracavitary brachytherapy for cervical cancer, demonstrating the feasibility and dosimetric advantages of this approach over conventional techniques.44,45 The procedure involves scanning patients after both intravenous delivery of [18F]FDG and insertion of tubes containing [18F]FDG into the tandem and colpostats. The PET image is then transferred to the 3D planning system, where volumes are delineated. Outcome data are awaited to assess the potential benefits of this approach.
SETUP UNCERTAINTY AND ORGAN MOTION
Several investigators have evaluated patient setup uncertainty in gynecology patients. The pelvis is among the most difficult body sites to setup accurately, even with sophisticated immobilization techniques.46




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree