Fig. 21.1
Axial computed tomography images showing isodose distributions for conventional (top left) and intensity-modulated radiation therapy (top right) plans for a patient with a gynecologic malignancy. The graph (bottom) demonstrates a comparison of the absolute volume of small bowel irradiated with the two plans [23]
While initially proposed to spare the bowel, bladder, and rectum, increasing interest has been focused more recently on the ability of IMRT to spare the pelvic BM. Brixey et al. was the first to report that IMRT planning could spare the pelvic BM, noting significant reductions in the volume of pelvic BM irradiated, particularly the BM in the iliac crests [27]. Interestingly, this reduction was achieved even though the pelvic BM was not intentionally avoided in the optimization process. Unsurprisingly, even greater BM sparing was seen by these same investigators when the iliac crests were included as an avoidance structure [28]. Mell and colleagues subsequently demonstrated that IMRT planning could reduce the dose to other pelvic BM sites, including the lumbosacral spine (Fig. 21.2) [29].


Fig. 21.2
Axial computed tomography images showing a comparison of isodose distributions for intensity-modulated (left), anteroposterior-posteroanterior (middle), and four-field conformal (right) radiotherapy plans in a patient with cervical cancer. The intensity-modulated plan provides superior conformity with lower dose to the bone marrow and normal tissue compared to the other two plans [29]
Comparable to studies focused on pelvic RT, several series have reported significant dosimetric benefits to IMRT planning in women treated with pelvic and para-aortic fields [30–34]. Portelance and colleagues at Washington University compared IMRT plans with traditional two- and four-field extended-field radiation therapy (EFRT) plans in 10 locally advanced cervical cancer patients [30]. Although comparable target coverage was seen, IMRT planning resulted in better sparing of the surrounding normal tissues. Compared to two-field EFRT plans, IMRT planning reduced the volume of the bowel, bladder, and rectum receiving the prescription dose by 61, 96, and 71 %, respectively. Compared to four-field EFRT plans, corresponding reductions were 60, 93, and 56 %, respectively. Lian and coworkers compared conventional EFRT plans in 10 endometrial cancer patients to various IMRT planning approaches [32]. Overall, IMRT planning resulted in superior target coverage and significant reductions in normal tissue doses. BM-sparing intensity-modulated EFRT (IM-EFRT) approaches have also been proposed [33, 34].
Data comparing conventional and IMRT planning in patients undergoing pelvic-inguinal irradiation and whole abdominal RT (WART) are more limited. Beriwal and coworkers at the University of Pittsburgh compared conventional and IMRT planning in 15 vulvar cancer patients undergoing pelvic-inguinal irradiation [35]. While target coverage was comparable, IMRT reduced the volume of the small bowel, bladder, and rectum receiving ≥ 30 Gy by 27 %, 26 %, and 41 %, respectively. Unfortunately, no significant difference was seen in the sparing of the femoral heads (an important organ at risk in these patients), perhaps due to the small patient numbers studied. Investigators at Memorial Sloan Kettering Cancer Center compared IMRT planning to conventional large field techniques in 10 endometrial cancer patients treated with WART [36]. IMRT planning resulted in better coverage of the peritoneal cavity, and a 60 % reduction in the volume of pelvic bones (a surrogate for pelvic BM) irradiated. Others have reported similar dosimetric benefits to IMRT planning in women undergoing WART [37].
Yang and coworkers performed a systematic literature review and meta-analysis of 13 published studies comparing conventional and IMRT planning in cervical or endometrial cancer patients treated using standard (non-escalated) doses [38]. Ten studies reported on the irradiated volume of small bowel, nine on rectum, eight on bladder, and six on BM. These series included 222 patients treated with IMRT and 233 undergoing conventional RT. The authors calculated the pooled average percent of irradiated volumes for various normal tissues and found statistically significant sparing of the rectum at doses of ≥ 30 Gy and the small bowel at doses ≥ 40 Gy. Overall, the pooled average irradiated volumes of the bladder and pelvic BM were consistently lower using IMRT than conventional planning, with the greatest differences seen at higher doses. None of these differences reached statistical significance. However, the small number of studies which included BM as an organ at risk limited the statistical power of this analysis.
21.3.2 Dose Escalation
The use of IMRT to deliver higher than conventional doses in gynecologic cancer patients has been the subject of multiple reports. Most attention to date has focused on patients with involved para-aortic lymph nodes, given the difficulty of safely delivering high doses to these sites using conventional techniques. Investigators at Washington University evaluated dose escalation to enlarged para-aortic lymph nodes using a simultaneous integrated boost (SIB) approach, whereby the doses to the involved and uninvolved nodes were 59.4 Gy (in 1.8 Gy fractions) and 50.4 Gy (in 1.53 Gy fractions), respectively [39]. On average, 97.6 % of the involved nodes received 100 % of the prescription dose, while the dose to surrounding normal tissues was maintained to acceptable levels. Ahmed and colleagues presented an alternative SIB approach whereby 60 Gy (in 2.4 Gy fractions) was delivered to involved para-aortic nodes, while the uninvolved nodal sites were simultaneously treated to 45 Gy (in 1.8 Gy fractions) (Fig. 21.3) [34]. Compared to both two- and four-field conventional approaches, IMRT resulted in reduced doses to the bilateral kidneys, spinal cord, and, at dose levels ≥ 40 Gy, BM. While IMRT planning reduced the volume of small bowel irradiated to high doses, the difference did not reach statistical significance.


Fig. 21.3
A coronal computed tomography image demonstrating the dose distribution for an extended-field intensity-modulated radiotherapy plan with a simultaneous integrated boost technique in a patient with cervical cancer with para-aortic nodal involvement. The involved para-aortic nodes were treated to 60 Gy (in 2.4 Gy fractions), while the uninvolved nodal sites were simultaneously treated to 45 Gy (in 1.8 Gy fractions) [34]
IMRT may also allow the delivery of higher than conventional pelvic doses in cervical cancer patients following hysterectomy. Patients with high-risk features (positive lymph nodes, positive margins, parametrial involvement), in particular, remain at risk of increased recurrence following surgery despite the delivery of conventional dose pelvic RT [40], suggesting a potential role for and benefit to dose escalation. D’Souza and colleagues compared conventional and IMRT treatment plans in 10 high-risk patients undergoing adjuvant pelvic RT and noted that IMRT planning maintained acceptable dose levels to the bowel, bladder, and rectum despite escalating the total pelvic dose from 45 to 54 Gy [41]. Du et al. explored the use of dose-escalated pelvic IMRT using the so-called reduced field IMRT approach, whereby 30 Gy was initially delivered to the uterus, cervix, upper vagina, paracervical and parametrial tissues, uterosacral region, and pelvic lymph nodes followed by a 30 Gy boost to the regional nodes and paracervical and parametrial tissues [42]. The IMRT plans were compared with conventional RT approaches covering the same target volumes; however, the total dose was intentionally limited to 45–55 Gy in the conventional RT group. Overall, the mean target volume dose was significantly higher in the IMRT patients (61.5 Gy vs 50.8 Gy, p = 0.046). Moreover, IMRT planning resulted in better dose conformity to the target and improved sparing of the rectum, bladder, and small intestine.
21.3.3 Brachytherapy Alternative/Replacement
The use of IMRT as a possible alternative to (or replacement for) brachytherapy is arguably one of the most controversial topics in all of gynecologic IMRT and has been the subject of multiple debates [43, 44]. While several authors have demonstrated that IMRT planning is capable of producing a pear-shaped dose distribution mimicking the dose distribution achieved with brachytherapy, integral normal tissue doses with brachytherapy are lower due to the steep dose falloff [45, 46]. Investigators at the Princess Margaret Hospital compared an IMRT boost with both a 3D conformal and a four-field box boost plan in 12 patients with cervical (8), endometrial (2), or vaginal (2) cancer who were not candidates for brachytherapy [47]. The authors found improved conformity with IMRT planning with a reduction of 22 and 19 % in the volume of rectum and bladder receiving the highest doses, respectively. Aydogan and colleagues compared IMRT and high-dose rate (HDR) brachytherapy planning in 10 endometrial cancer patients and noted better dose uniformity and improved bladder and rectal sparing with IMRT (Fig. 21.4) [48]. In select unfavorable anatomy cases, IMRT planning may even result in better target coverage [49]. In contrast, Sharma and colleagues compared IMRT and interstitial brachytherapy plans in 12 locally advanced cervical cancer patients and found that brachytherapy resulted in better target coverage and an improved conformity index [50].


Fig. 21.4
Axial computed tomography images with dose distributions for a high-dose rate vaginal cylinder brachytherapy (top) and intensity-modulated radiotherapy (bottom) plan in a patient with endometrial cancer. The intensity-modulated plan provides superior dose conformity and improved bladder and rectal sparing at higher isodose levels [48]
As opposed to the above approaches in which an IMRT boost is delivered after the completion of external beam RT, Guerrero and coworkers proposed a novel SIB technique whereby the boost is incorporated into the initial treatment [51]. As envisioned, the regional pelvic nodes receive 45 Gy in 1.8 fractions, while the cervical tumor is treated to 70–77.5 Gy in 2.8–3.1 Gy fractions, which is felt to be radiobiologically equivalent to 45 Gy whole pelvic RT followed by 30 Gy HDR brachytherapy. The SIB approach achieved better bowel and bladder sparing than a sequential IMRT boost, with significant shortening of the overall treatment course. While appealing, a concern with this approach is that the decision not to use brachytherapy must be made at diagnosis. There may be some patients in whom brachytherapy would never be feasible, but in most, it is preferable to assess the clinical response to pelvic RT prior to deciding on whether or not to perform brachytherapy.
IMRT has also been proposed as a possible adjunct to brachytherapy. Assenholt et al. evaluated an applicator-guided IMRT boost combined with brachytherapy to improve coverage of large or topographically unfavorable tumors [52]. IMRT planning was able to improve but did not replace the dose given by intracavitary brachytherapy. Duan and coworkers compared conventional brachytherapy, optimized brachytherapy, and optimized brachytherapy with an IMRT boost and found that HDR brachytherapy plus IMRT achieved better target coverage where conventional brachytherapy dose was suboptimal [53]. Others have proposed using an SIB-IMRT approach in bulky cervical cancers prior to brachytherapy [54].
21.4 Clinical Outcome Studies
A large (and growing) number of clinical studies have been published in recent years evaluating the outcome of gynecologic cancer patients treated with IMRT. By far, the lion’s share has been single institution reports focusing on cervical and/or endometrial cancer patients treated with conventional dose pelvic IMRT. However, an increasing number of reports have included patients treated with more comprehensive fields, with dose-escalated techniques or with IMRT in lieu of brachytherapy. Moreover, outcome studies have also begun to appear using IMRT in other tumor sites, including vaginal and ovarian cancers.
21.4.1 Pelvis
In a series of reports [55, 56], Mundt and colleagues at the University of Chicago were the first to report on the outcome of gynecologic cancer patients treated with pelvic IMRT using conventional doses. Their initial study focused on a mixed cohort of 40 cervical and endometrial cancer patients undergoing pelvic IMRT (median dose, 45 Gy) either following surgery or as definitive treatment. Compared to 40 patients treated with conventional techniques, IMRT patients experienced less grade ≥ 2 acute GI toxicity (60 % vs. 91 %, p = 0.002). Although less grade ≥ 2 GU toxicity was seen in the IMRT group (10 % vs. 20 %), this difference did not reach statistical significance (p = 0.22). In a follow-up report [57], these investigators compared rates of chronic GI toxicity in 36 IMRT and 30 conventional RT patients. The groups were well balanced in terms of age, site, stage, chemotherapy, radiation dose, and brachytherapy, except for a higher frequency of surgery in the IMRT group. Overall, IMRT patients had a lower rate of chronic GI toxicity (11 % vs. 50 %, p = 0.001) (Fig. 21.5). On multivariate analysis, this reduction remained statistically significant (p = 0.01).


Fig. 21.5
A comparison of chronic gastrointestinal toxicity in gynecologic cancer patients treated with intensity-modulated (gray bars) and conventional radiotherapy (black bars). Overall, the patients treated with intensity-modulated radiation therapy had a lower rate of chronic gastrointestinal toxicity (11 % vs. 50 %, p = 0.001) [57]
Hasselle and coworkers recently presented the combined experience of the University of Chicago and the University of California San Diego (UCSD) using pelvic IMRT in cervical cancer patients [58]. Overall, 111 patients underwent pelvic IMRT between 2000 and 2007 either following surgery (22) or combined with chemotherapy and intracavitary brachytherapy (89). Overall, 95 patients (86 %) received concomitant chemotherapy and 71 (80 %) of the definitive patients underwent brachytherapy. At a median follow-up of 27 months, the three-year actuarial overall survival (OS) and disease-free survival (DFS) rates of the entire group were 78 % and 69 %, respectively. The three-year pelvic control rates for stage IB–IIA and stage IIB–IVA patients undergoing definitive treatment were 94.7 % and 70.8 %, respectively. Moreover, the actuarial three-year pelvic control in the postoperative patients was 100 %. Overall treatment was well tolerated; estimates of acute and late grade ≥ 3 GI or GU toxicities were 2 % and 7 %, respectively.
Investigators at Washington University have also reported on their experience using IMRT in cervical cancer patients, comparing the outcomes of 135 patients undergoing IMRT (primarily pelvic IMRT) with those seen in 317 treated with conventional RT [59]. Controlling for a variety of clinical and treatment factors, the IMRT patients were found to have a better cause-specific survival (CSS) (p < 0.0001) and OS (p < 0.0001) than the conventional RT patients. A nonsignificant trend favoring IMRT was also seen between the two groups in terms of recurrence-free survival (p = 0.07). Patients treated with IMRT developed fewer grade ≥ 3 late GI or GU sequelae (6 % vs. 17 %, p = 0.002). It should be noted that the differences in OS and CSS were unexpected and potentially biased by other differences between the groups. Furthermore, it is difficult to attribute these differences in survival to treatment technique alone given the similar pelvic recurrence rates. The initial Washington University experience using adjuvant pelvic IMRT in endometrial cancer was published earlier [60]. Overall, 19 women with stages IB–IVB endometrial cancer received pelvic IMRT following surgery. None developed acute grade ≥ 3 acute GI or GU toxicity.
More recently, investigators at the Memorial Sloan Kettering Cancer Center have published their experience using adjuvant pelvic IMRT in patients with cervical and endometrial cancer. Folkert et al. treated 34 high-risk cervical cancer patients following surgery with a median total dose of 50.4 Gy [61]. All patients received concomitant cisplatin. At a median follow-up of 44 months, the five-year actuarial OS and DFS rates of the entire group were 91.1 % and 91.2 %, respectively. Grade ≥3 acute GI and GU sequelae occurred in only 2.9 % and 0 %, respectively. No patients developed grade ≥3 chronic toxicity. In a separate report, Shih and colleagues treated 46 stages I–III endometrial cancer patients with adjuvant pelvic IMRT (median dose, 50.4 Gy) and in 30 patients (66 %) with adjuvant chemotherapy [62]. At a median follow-up of 52 months, the five-year actuarial DFS and OS rates were 88 % and 97 %, respectively. Only two patients developed grade 3 or higher GI toxicity (one acute and one chronic). No significant GU acute or chronic toxicity was seen. Others have reported favorable outcomes using adjuvant pelvic IMRT in cervical and endometrial cancer patients [63–65].
Considerable experience using pelvic IMRT in gynecologic cancer patients has been reported from centers in Asia [66–70]. Chen et al. at the Taichung Veterans General Hospital in Taiwan treated 109 stage IB–IVA cervical cancer patients with IMRT (88 % pelvic IMRT) combined with weekly chemotherapy [66]. At a median follow-up of 32.5 months, the three-year DFS and OS rates were 67.6 % and 78.2 %, respectively. Three patients (2.7 %) developed grade ≥3 acute GI toxicity. Late grade 3 or higher GI and GU sequelae developed in 4.6 % and 6.4 % of patients, respectively. In a subsequent report, the risk of late complications in 83 IMRT and 237 non-IMRT intact cervical cancer patients was compared [67]. Overall, IMRT patients had lower rates of grade ≥2 (23 % vs. 30 %) and grade ≥3 (8 % vs. 12 %) GI or GU late sequelae; however, neither difference reached statistical significance (p = 0.24 and p = 0.33). Other investigators in Asia have reported improved toxicity profiles using adjuvant pelvic IMRT in cervical cancer patients undergoing pelvic irradiation following surgery [69].
Chen and colleagues have also published an analysis of 101 stage IA–IIIC2 endometrial cancer patients treated with either IMRT (65) or conventional (36) RT at their institution and noted significant reductions in GI and GU toxicity using IMRT [68]. Rates of grades 2 and 3 acute GI and GU toxicity in the conventional group were 55.6 % and 11.1 % and 19.4 % and 8.3 %, respectively. Corresponding rates in the IMRT group were 27.7 % and 6.2 % and 16.9 % and 0 %, respectively. Grade ≥3 late GI and GU toxicities were seen in 2.8 % and 2.8 % of the conventional RT group. No grade ≥3 late toxicities developed in the IMRT patients. No differences were seen in terms of DFS, OS, or local control between the two groups. In contrast, a recent SEER study from the United States found a comparable rate of GI and GU sequelae between endometrial cancer patients receiving adjuvant pelvic IMRT and those treated with conventional techniques, except for a higher rate of bowel obstruction in the IMRT group [7]. However, details about the quality of the IMRT plans or extent of nodal surgery in the two groups were not known, both of which may have influenced the results.
While most published reports to date of pelvic IMRT have been in patients with cervical and endometrial cancers, emerging data have appeared in other disease sites [70–72]. Investigators at Stanford University treated 10 vaginal cancer patients with IMRT (predominantly pelvic IMRT) combined in most patients with concomitant chemotherapy and intracavitary brachytherapy [72]. None of these patients developed a locoregional recurrence or experienced grade 3 or higher toxicity. The experience at MD Anderson Cancer Center treating vaginal cancers unable to undergo brachytherapy with IMRT alone is discussed later in this chapter [73].
Despite the observation that patients undergoing pelvic IMRT and chemotherapy often experience less hematologic toxicity even when the BM is not included in the optimization process [27], hematologic toxicity rates remain high in most series which do not intentionally spare the BM [61, 64, 65, 68] or, at best, only modestly reduce the BM dose [60]. For example, Chen and colleagues treated 68 high-risk cervical cancer patients with chemotherapy and pelvic RT planned using either conventional (35) or IMRT (33) techniques [66]. The pelvic BM was not included in the IMRT planning. Grade 2 and 3 hematologic sequelae occurred in 31 % and 9 %, respectively, of the conventional RT patients. Corresponding rates in the IMRT patients were 27 % and 6 %, respectively (p = 0.72). In contrast, Du and coworkers included BM as a constraint in the planning process and found less grade 2 or higher leukopenia (4 % vs. 10 %, p = 0.026) compared to that seen in patients treated with conventional techniques [42]. Efforts to optimize BM-sparing approaches in patients undergoing pelvic IMRT or more comprehensive fields are discussed further in the subsequent section.
Limited published data are available on the use of dose-escalated pelvic IMRT approaches. The report from Du et al. described earlier is the largest experience to date [42]. They found that patients treated with dose-escalated IMRT had lower rates of proctitis (p = 0.001), enteritis (p = 0.03), cystitis (p = 0.001), and dermatitis (p = 0.04) compared to a control group of 60 conventional pelvic RT patients (treated without dose escalation). The utility of dose-escalated pelvic IMRT is the subject of an ongoing phase II clinical trial in India [74]. Others have explored the use of SIB techniques to escalate dose to either the cervix [49] or involved lymph nodes [75].
Schwarz and colleagues performed a feasibility study of IMRT (92 % pelvic IMRT) in 24 postoperative high-risk cervical cancer patients [76]. Eighteen (75 %) received concomitant chemotherapy; all underwent vaginal brachytherapy. Patients received higher than conventional pelvic doses (median dose, 51.2 Gy); two patients with gross disease underwent local boosts (10–15 Gy). Organs at risk included in the optimization process included bladder, bowel, and pelvic bones (surrogate for BM). IMRT and brachytherapy were completed as planned in all patients. Acute grade 3 GI toxicity occurred in 50 % of patients (five anorexia, four diarrhea, three nausea), predominantly in patients receiving concomitant chemotherapy. Only one patient developed a grade 3 acute GU toxicity. Grade 3 and 4 hematologic sequelae occurred in 63 % and 21 % of concurrent chemoradiotherapy patients, respectively.
Investigators from Chiang Mai Hospital in Thailand enrolled 15 locally advanced cervical cancer patients on a prospective feasibility study of pelvic IMRT and image-guided brachytherapy [77]. All patients received 45 Gy in 1.8 Gy daily fractions with concurrent cisplatin followed by image-guided brachytherapy (7 Gy × 4 prescribed to the high-risk CTV). All patients completed treatment as planned. Most acute toxicities were mild with no patients developing grade ≥3 acute sequelae. At a median follow-up of 14 months, only one patient has developed a local recurrence. No significant late sequelae were noted.
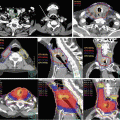
Investigators at UCSD enrolled 31 patients (19 gynecologic cancers) on a prospective trial evaluating the ability of IMRT to reduce dose to functional BM, as opposed to constraining dose to the total pelvic BM [78]. Functional BM was identified using both 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/computed tomography (CT) and an investigational magnetic resonance imaging (MRI) protocol, IDEAL IQ, which identifies regions of high BM cellularity. The intersection of BM subregions with SUV values above the mean and those with the fat fraction below the mean was used to identify functional BM sites (Fig. 21.6). Two types of IMRT plans were compared: standard BM-sparing plans in which the total BM (using the pelvic bones as a surrogate) was included in the optimization process and functional BM-sparing plans in which only the functional BM subregions were included. In the 19 gynecology patients, the mean functional BM V10 and V20 were 85 % vs. 94 % (p < 0.0001) and 70 % vs. 82 % (p < 0.0001), respectively, for functional BM-sparing IMRT versus standard BM-sparing IMRT. Of 10 subjects treated with functional BM-sparing techniques with concomitant chemotherapy, three (30 %) experienced acute grade ≥3 hematologic toxicity.


Fig. 21.6
Axial, coronal, and sagittal views of fat fraction maps (a) of pelvic bone marrow (BM) in a cervical cancer patient before treatment. Segmented functional BM regions are superimposed onto the simulation computed tomography images (b) [78]
Mabuchi and colleagues presented a phase I trial in high-risk cervical cancer patients treated with adjuvant pelvic IMRT (50.4 Gy) and concurrent weekly carboplatin and escalating doses of paclitaxel (initially 35 mg/m2 and increasing by 5 mg/m2 intervals) [79]. Nine women were enrolled with dose-limiting toxicity (DLTs) occurring at the second dose level (40 mg/m2/week). No patients treated with 35 mg/m2/week developed dose-limiting toxicity. Two of the three DLTs were hematologic.
In 2006, the RTOG launched a prospective phase II trial (RTOG 0418) evaluating adjuvant pelvic IMRT in endometrial and cervical cancer patients. The prescribed dose in all patients was 50.4 Gy in 1.8 Gy daily fractions; however, while the endometrial cancer patients received IMRT alone, the cervical cancer patients received concomitant weekly cisplatin. The results of the cervical [80] and endometrial [8, 81] cancer patients have been reported separately. Overall, 53 endometrial cancer patients were eligible for analysis. While the primary endpoint of reproducibility of the IMRT approach was achieved, considerable treatment planning deviations were noted. The proportion of cases in which doses to critical normal tissues exceeded protocol criteria were bladder (67 %), rectum (76 %), bowel (17 %), and femoral heads (33 %). Seven cases (17 %) had a dose to the small bowel that exceeded the prescribed dose constraints by >10 %. Overall, a nonsignificant absolute reduction of 12 % was seen in acute GI toxicity compared to historical controls. However, the trial was not powered to detect such a difference. It thus remains unclear whether a significant difference could have been detected in a larger cohort. In a separate abstract, these investigators reported excellent tumor control rates in these patients, with a three-year OS and DFS of 91 % and 91 %, respectively [8].
The outcome of the cervical cancer patients on RTOG 0418 has only been presented to date in abstract form [80]. Forty patients were eligible for analysis, all of whom received pelvic IMRT plus concomitant chemotherapy. With a median follow-up of 2.7 years, the two-year actuarial DFS and OS rates were 86.9 % and 94.6 %, respectively. Two-year actuarial local recurrence and distant-metastasis rates were 10.6 % and 10.3 %, respectively. In an earlier abstract [82], the authors noted that grade ≥2 bowel toxicity occurred in 22.5 % of patients which was statistically significantly lower than the hypothesized rate of 40 % (p = 0.04).
Barillot and coworkers presented the results of a prospective phase II trial evaluating adjuvant pelvic IMRT in patients with stage IB (grade 3), IC, or II endometrial cancer [10]. A total of 46 patients were eligible for analysis. All patients received adjuvant pelvic IMRT to a dose of 45 Gy in 25 fractions. Thirty-six patients (75 %) received an additional vaginal vault HDR brachytherapy boost. The primary endpoint was the incidence of acute grade ≥2 GI toxicity. Thirteen patients (27 %) developed at least one acute grade 2 GI toxicity. There were no ≥ 3 GI toxicities observed. Nine patients (19 %) developed grade 2 GU toxicity. The authors noted that the incidence and timing of the GI toxicity were similar to those reported in RTOG 0418 [81].
Two prospective randomized trials both from India have been published evaluating pelvic IMRT in gynecologic patients (Table 21.1) [74, 83]. Gandhi and colleagues performed a phase III randomized trial in 44 stage IIB–IIIB squamous cell cervical cancer patients radiotherapy [83]. Patients received 50.4 Gy in 28 Gy fractions delivered with either conventional or IMRT with concomitant chemotherapy. All patients subsequently underwent brachytherapy (7 Gy × 3, Point A). While no difference was seen in the median treatment duration (9.1 weeks in both arms), the only patients who had treatment breaks and/or delays due to diarrhea or low blood counts were in the conventional group. IMRT patients had less grade ≥2 acute GI toxicity (32 % vs. 64 %. p 0.03) and less grade ≥2 emesis (9 % vs. 36 %, p = 0.03). No difference was seen in the frequency of either acute GU or hematologic toxicity. At a median follow-up of 21.6 months, the incidence of grade ≥2 chronic GI toxicity was also lower in the IMRT group (5 % vs. 23 %, p = 0.011). No differences were seen in DFS or OS rates between the two groups.
Table 21.1
Randomized trials comparing pelvic intensity-modulated radiation therapy to conformal radiation therapy in patients with cervix cancer
Author | Number of patients | Phase | Median follow-up (mos.) | Results |
|---|---|---|---|---|
Gandhi et al. [83] | 44 | III | 21 | No difference in OS or DFS; less grade ≥2 acute and late GI toxicity in IMRT arm; no difference in GU or hematologic toxicity |
Shrivastava et al. [74] | 86 | IIB | 17 | Less acute high grade GI and hematologic toxicity with IMRT despite dose escalation |
Shrivastava and colleagues presented the preliminary results of a phase IIB randomized trial comparing conventional pelvic (40 Gy in 20 fractions) with dose-escalated pelvic IMRT (50 Gy) [74]. All 86 patients received concomitant chemotherapy and planned HDR brachytherapy. No differences were seen in terms of compliance or response between the two treatment arms.
However, despite the escalation of the pelvic dose, the IMRT patients experienced fewer high grade GI and hematologic toxicities. Two IMRT patients developed grade 3 GU toxicity. At a median follow-up of 17 months, six conventional and three IMRT patients have developed recurrent disease. Patients are continuing to be observed for late toxicities and disease recurrence.
Currently, several multicenter prospective clinical trials include and/or are assessing IMRT in gynecologic cancer patients. While earlier cooperative groups trials conducted by the GOG and RTOG previously did not allow IMRT, it is now permitted in several ongoing studies including GOG 0249, GOG 0258, GOG 0263, and RTOG 0724. Based on the favorable results of RTOG 0418, the RTOG has initiated the RTOG 1203 trial, which randomizes postoperative endometrial and cervical cancer patients to conventional or pelvic IMRT. Mell and colleagues have recently launched the International Evaluation of Radiotherapy Technology Effectiveness in Cervical Cancer (INTERTECC) trial [9], a phase II/III trial designed to test the efficacy of IMRT in the treatment of cervical cancer patients, for both definitive and adjuvant approaches.
21.4.2 Pelvis and Para-aortic Nodes
Multiple investigators have reported on the outcome of gynecologic cancer patients treated with IM-EFRT. Liang and colleagues presented their experience using prophylactic IM-EFRT in 32 stage IB2–IIIB cervical cancer patients with positive pelvic and negative para-aortic lymph nodes [84]. The prescribed dose to the para-aortic region was 40 Gy in 25 fractions. All patients received concurrent cisplatin chemotherapy and brachytherapy. Acute grade ≥ 3 GI and GU toxicities were seen in 6.2 % and 3.1 % of patients, respectively. Two grade 3 late sequelae were noted (one GI and one GU). Compared to historical controls, the IMRT patients demonstrated improved three-year actuarial OS (87 % vs. 62 %, p = 0.02), DFS (82 % vs. 54 %, p = 0.02), and distant-metastasis free survivals (79 % vs. 57 %, p = 0.01). Others have reported similarly favorable outcomes in patients treated with prophylactic IM-EFRT [33].
Several investigators have explored dose-escalated IM-EFRT approaches, using either sequential [85–88] or integrated [33, 88, 89] boost techniques. Salama et al. treated 13 endometrial or cervical cancer patients with IM-EFRT [85]. All initially received 45 Gy to the pelvis/para-aortic regions with concomitant chemotherapy followed by a boost of 9 Gy to the involved para-aortic nodes. No patients experienced grade ≥3 acute GI or GU toxicity. At a median follow-up of 11 months, the one-year actuarial in-field control rate was 90 %. Jensen et al. updated this experience with a total of 21 patients and reported an 18-month rate of locoregional control of 90 % [86]. Acute grade ≥3 GI and GU toxicity occurred in four and zero patients, respectively. The two-year incidence of late grade ≥3 GU toxicity was 4.8 %. No patients experienced grade ≥3 late GI toxicity. Beriwal et al. treated 36 stage IB2–IVA cervical cancer patients with IM-EFRT (45 Gy) using an SIB boost approach to boost involved para-aortic nodes to 55–60 Gy in 2.2–2.4 Gy daily fractions [88]. At a median follow-up of 18 months, the two-year actuarial locoregional control, DFS, OS, and grade ≥3 toxicity rates for the entire group were 80 %, 51 %, 65 %, and 10 %, respectively. Acute grade ≥3 GI and GU toxicities occurred in 2.8 % and 2.8 % of patients, respectively.
Investigators at the Brigham and Women’s Hospital reported on the outcomes of 32 gynecologic cancer patients (22 endometrial, 10 cervical cancer) who underwent sequential IMRT boosts to involved lymph node regions and concurrent chemotherapy [89]. Twelve patients had pelvic nodal boosts, 13 para-aortic nodal boosts, and 7 both. The median nodal size was 2.5 cm (range, 1.4–4.2 cm), and the median total dose was 63 Gy (range, 54–68.4 Gy). At a median follow-up of 21.8 months, the two-year nodal control was 85 %. Treatment was well tolerated, with a two-year actuarial late grade ≥3 toxicity rate of 14 %.
Marnitz and colleagues evaluated the feasibility of a SIB-IMRT approach in cervical cancer patients with positive pelvic and/or para-aortic lymph nodes undergoing RT and chemotherapy [75]. Of 40 patients, 29 (72.5 %) had documented enlarged lymph nodes and underwent pre-RT lymph node dissections. IMRT plans were generated to treat the pelvis and/or para-aortic region to 50.4 Gy (in 1.8 Gy fractions) and involved lymph nodes to 59.36 (in 2.12 Gy fractions). All patients received concurrent chemotherapy, predominantly weekly cisplatin. Overall, treatment was well tolerated with only two grade 3 acute GI toxicities (one diarrhea, one nausea) and no grade 3 GU toxicity. Tumor control rates were not reported. Others have reported favorable results using dose-escalated IMRT as salvage therapy in women with recurrent disease in the para-aortic region [90].
Prospective trials have also been performed in para-aortic node-positive cervical cancer patients [91] and in endometrial cancer patients undergoing IMRT in lieu of brachytherapy [92]. Investigators at the Shandong Tumor Hospital and Institute completed a novel trial in 60 para-aortic positive cervical cancer patients who were serially assigned to either conventional dose (45–50 Gy) conformal para-aortic irradiation or high-dose (58–68 Gy) IMRT. Overall, IMRT patients experienced less acute grade ≥3 myelosuppression (4 % vs. 19 %, p = 0.005), dermatitis (0 % vs. 6 %, p = 0.04), and GI toxicity (4 % vs. 19 %, p = 0.005) and a higher complete response rate (57 % vs. 28 %, p = 0.02) than the conventional RT patients. At a median follow-up of 28 months, the three-year OS rate was superior in the IMRT group (36 % vs. 16 %, p = 0.016). Chronic enterocolitis was lower in the IMRT group (0 % vs. 19 %, p = 0.001).
21.4.3 Pelvis and Inguinal Nodes
The sole published experience using pelvic-inguinal IMRT in gynecologic cancer patients is from the University of Pittsburgh. In a series of reports [35, 93], Beriwal et al. presented the outcomes of locally advanced vulvar cancer patients undergoing preoperative pelvic-inguinal IMRT combined with chemotherapy followed by planned surgery (Fig. 21.7). In their most recent report [93], 42 stage I–IVA patients, all of whom required preoperative treatment, were treated with a modified Gynecologic Oncology Group (GOG) regimen of 5-fluorouracil and cisplatin with twice-daily IMRT (36) or weekly cisplatin with daily IMRT (6). The twice-daily IMRT regimen consisted of 1.6 Gy twice daily for 10 fractions, followed by 1.8 Gy daily for 7 or 8 days, followed by a planned break of 10 days, and then resumption of radiation with 1.6 Gy twice daily for 10 more fractions. The patients who underwent daily IMRT received 50.4 Gy in 1.8 Gy fractions. Surgery was performed 6–10 weeks following treatment. Overall, treatment was well tolerated with all patients completing IMRT and chemotherapy as planned. No acute grade 3 or higher GI or GU toxicities occurred. One patient developed grade 3 cutaneous toxicity. Of 41 evaluable patients, a complete clinical response was noted in 21 (51.2 %). Thirty-three patients underwent surgery of which 16 (48.5 %) had a pathologic complete response in the vulva. Of these, 15 (93.8 %) remained without disease recurrence. Of the 17 who had a pathologic partial response, 8 (47.1 %) developed a local recurrence. No patient in the series developed a grade 3 or higher late GI or GU complication.


Fig. 21.7
Axial computed tomography images showing isodose distributions for an intensity-modulated radiation therapy plan covering the inguinal (left) and pelvic (right) lymph nodes in a patient with locally advanced vulvar cancer undergoing preoperative radiotherapy [35]
21.4.4 Whole Abdomen
Given the decreasing use of WART in the United States and other countries, it is not surprising that clinical studies in gynecologic cancer patients undergoing intensity-modulated WART (IM-WART) are limited [91–93]. Mahantshetty et al. at Tata Memorial Center presented the outcomes of 8 relapsed ovarian cancer patients with disease confined to the abdomen and/or pelvis treated with salvage intensity-modulated WART [92]. Using an SIB approach, 25 Gy in 1 Gy fractions was delivered to the whole abdomen, while the pelvis received 45 Gy in 1.8 Gy fractions (Fig. 21.8). Treatment was well tolerated with no patient requiring significant unplanned treatment breaks. Overall, three patients developed grade 2 GI and two developed grade 2 transient liver toxicities. While all patients had been heavily pretreated with chemotherapy, only three (37.5 %) developed grade ≥3 hematologic toxicity. No grade ≥3 GI or renal toxicity was noted. At a median follow-up of 15 months, three patients progressed in the abdomen and/or pelvis. The other 5 remained free of disease.


Fig. 21.8
Axial (a), coronal (b), and sagittal (c) computed tomography images showing dose distributions from a helical tomotherapy plan used for a patient with relapsed ovarian cancer [94]
Rochet and coworkers presented a feasibility trial evaluating chemotherapy followed by IM-WART in newly diagnosed ovarian cancer patients [94]. Ten optimally debulked stage IIIC ovarian cancer patients were enrolled and received six cycles of carboplatin/paclitaxel chemotherapy followed by IM-WART (30 Gy in 1.5 Gy daily fractions). Overall, treatment was well tolerated with only one patient developing an acute grade 3 GI toxicity. Three patients experienced grade 3 leukopenia. While no patient developed chronic enteritis, three patients required surgery due to small-bowel obstruction (one of which was noted to have an abdominal recurrence). At a median follow-up of 23 months, the two-year actuarial DFS, OS, and local progression free survival rates were 63, 68, and 78 %. These results compared favorably to earlier studies of carboplatin/paclitaxel followed by conventional WART [95].
21.4.5 Brachytherapy Alternative/Replacement
Several investigators have reported on the outcome of gynecologic cancer patients treated with IMRT in lieu of brachytherapy [96–101]. The largest series to date was presented by Huang and coworkers at the Princess Margaret Hospital and consisted of 70 locally advanced/recurrent gynecologic cancer patients (77 % cervical cancer) who were deemed ineligible for brachytherapy, primarily due to unfavorable anatomy or tumor bulk [96]. Treatment consisted of 12–30 Gy delivered in 1.4–2 Gy/fraction. Acute grade ≥3 non-hematologic toxicities were infrequent. Late grade ≥3 sequelae included cystitis (2.8 %), enteritis (1.4 %), and fistula (1.4 %). At a median follow-up of 1.33 years, the three-year actuarial OS and DFS rates were 84.4 % and 49 %, respectively. Overall, pelvic, retroperitoneal, and distant recurrences occurred in 39, 27, and 27 % of patients. Olson and coworkers reported on 32 endometrial cancer patients treated with pelvic RT followed by either brachytherapy (24) or an IMRT/conformal (8) boost [99]. At a median follow-up of 18.6 months, no difference was seen between the OS and CSS of the brachytherapy and non-brachytherapy groups. Moreover, no difference was seen in terms of acute toxicity.
Hypofractionated IMRT boost techniques in brachytherapy ineligible patients have also been reported [97, 98, 100, 101]. Hsieh and colleagues treated nine stage IIB–IVA cervical cancer patients in whom brachytherapy was felt not to be feasible with an IMRT boost of 16–27 Gy in 5–9 fractions [101]. Treatment was well tolerated with no grade ≥3 acute toxicities. Only one patient developed a late grade ≥2 toxicity. The three-year actuarial OS, DFS, and local recurrence-free survivals were 46.9, 25.9, and 77.8 %. Molla et al. reported on 16 patients treated with a hypofractionated boost (94 % IMRT) for definitive (4 Gy × 5 fractions) or adjuvant therapy (7 Gy × 2 fractions) (Fig. 21.9) [97]. At a median follow-up of 12.6 months, only one patient (6.2 %) failed locally. Treatment was well tolerated with no grade ≥3 acute sequelae and only one late GI toxicity. In a follow-up report, these investigators updated their experience in 26 postoperative patients [98]. At a median follow-up of 47 months, the three-year local control and OS rates were 96 % and 95 %, respectively. No grade ≥3 acute or chronic sequelae were seen. Kemmerer et al. treated 11 endometrial cancer patients with a 6 Gy × 5 twice weekly boost (82 % IMRT). At 18 months, the OS and DFS rates were 57 % and 68 %, respectively [100].


Fig. 21.9
Computed tomography images showing dose distributions for dynamic-arc (left) and fixed-field intensity-modulated radiation therapy (right) hypofractionated boost plans used for gynecologic cancer patients unable to undergo brachytherapy [97]
The sole series using IMRT in place of brachytherapy in patients with vaginal cancer was presented by investigators from the MD Anderson Cancer Center [73]. Twenty-three patients (eight stages I–II, 15 stages III–IVB) ineligible for brachytherapy received treatment with IMRT alone. The total dose including pelvic RT (when given) was 65.1 Gy (range, 61–70 Gy). At a median follow-up of 35 months, the five-year OS rates for stage I–II, III, IVA, and IVB patients were 100 %, 82 %, 28 %, and 0 %, respectively. Five-year pelvic control rates for stages I, II, III, IVA, and IVB patients were 100 %, 66 %, 86 %, 50 %, and 0 %, respectively.
Macchia et al. performed a prospective phase I trial in 12 stage IB–IC endometrial cancer patients undergoing IMRT in place of vaginal brachytherapy [92]. Two dose levels were evaluated: 5 Gy × 5 and 6 Gy × 5. No patients at either dose level experienced a DLT. No grade 2 or higher late toxicity was noted. The authors are now evaluating the efficacy of the higher dose level in a phase II trial.
21.5 Technical Issues and Considerations
21.5.1 Patient Selection
When IMRT was first introduced, only a subset of gynecologic cancer patients was considered eligible at many centers. By far, the most commonly treated patients were cervical and endometrial cancer patients undergoing adjuvant pelvic RT. However, even among this group, exceptions existed, notably markedly obese patients due to the inability to obtain full external contours and concerns over setup accuracy [56]. Over time, however, indications for gynecologic IMRT have grown considerably, and nearly all patients are now considered candidates, even those treated with comprehensive treatment volumes as well as women with gross residual disease requiring higher than conventional doses.
Considerable controversy has long existed regarding the use of IMRT in intact cervical cancer patients [4, 102]. In fact, at many prestigious centers, IMRT is commonly used in postoperative patients but not in women with intact disease. Multiple concerns are often cited; however, the major issue is clearly internal organ motion. Nevertheless, such concerns can be addressed with the use of proper treatment margins and daily in-room imaging. Moreover, the favorable published outcome results from experienced investigators clearly support the use of IMRT in these patients [58, 59, 66].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree