and Zdeněk Fryšák1
(1)
Department of Internal Medicine III – Nephrology, Rheumatology and Endocrinology, Faculty of Medicine and Dentistry, Palacky University Olomouc and University Hospital Olomouc, Olomouc, Czech Republic
Keywords
Hashimoto’s ThyroiditisHypoechoic micronodulesFibrous bandsGoiterAtrophic glandHyperechoic fibrous septaHTAtrophic thyroid glandSporadic hypoechoic micronodulesTransient thyrotoxicosisColor flow Doppler ultrasound3.1 Hashimoto’s Thyroiditis: Chronic Lymphocytic Thyroiditis
3.1.1 Essential Facts
Hashimoto’s thyroiditis (HT), also known as chronic lymphocytic thyroiditis (CLT) or autoimmune thyroiditis (AT), is the most frequent cause of hypothyroidism and goiter in iodine sufficient areas [1].
In addition to classic form, several other clinico-pathological entities are now included under the term of HT: fibrous variant, IgG4-related variant, juvenile form, Hashitoxicosis, and painless thyroiditis (sporadic or post-partum) [2].
Japanese pathologist Hakaru Hashimoto first described the disease as “struma lymphomatosa” in 1912. But the dominant German school of thought in medicine asserted this histology as an early phase of Riedel’s thyroiditis. Hashimoto’s description was then ignored and forgotten. In 1931, however, Allen Graham et al. reported struma lymphomatosa and endorsed Hashimoto’s conclusion that it was a disease in its own right. Since then, this disease has been eponymously referred to as “Hashimoto’s thyroiditis.” In 1956 Doniach et al. purified a thyroglobulin antibody (Tg-Ab) from the sera and proposed that HT is an autoimmune disease. In 1962 the concept of HT as organ-specific autoimmune disease was established [3].
HT is one of the most common organ-specific autoimmune diseases. Its annual incidence is estimated to be between 0.3 and 1.5 cases per 1000 persons, with no significant race-related predominance [1].
HT affects 1.3% of children and has a female predominance [4].
Pathologically proven HT is defined as the presence of diffuse lymphocytic infiltration with occasional germinal centers, small thyroid follicles with sparse colloid, and fibrosis [5].
The diagnosis of HT is defined as elevated thyroid peroxidase (TPO-Ab) and thyroglobulin antibodies (Tg-Ab). High serum TPO-Ab concentrations are present in more than 90–95% of patients [6].
In the total population, positive TPO-Ab is detected in 13.0 ± 0.4%, and positive Tg-Ab in 11.5 ± 0.5%. The prevalence of positive antibodies is lower in the disease-free population: TPO-Ab, 11.3 ± 0.4% and Tg-Ab, 10.4 ± 0.5%. The prevalence of positive TPO-Ab and positive Tg-Ab in the total and disease-free population is higher in women than in men and increases with age, especially among women [7].
Clinical findings: the presence of a firm, painless, diffusely enlarged gland; a large number of patients with lymphocytic thyroiditis have nodular enlargement [8].
Long-standing HT causes shrinking and atrophy of the thyroid, but may also lead to diffuse enlargement of the gland and/or formation of nodules. These nodules should be differentiated from PTC and primary thyroidal non-Hodgkin lymphoma, which are possible complications of HT [9].
Autoimmune thyroid diseases (AITD) like Graves’ disease (GD) and HT are complex diseases in which autoimmunity against the thyroid autoantigens develops at a certain genetic background, provoked by exposure to environmental factors. Genetic factors contribute about 70–80% to the pathogenesis, and environmental factors (including infection, stress, iodine, selenium, vitamin D and smoking) contribute about 20–30% to the pathogenesis. Blood relatives of AITD patients carry a risk to contract AITD themselves [10].
Prevalence of the two major AITD (GD and HT), which are characterized by thyrotoxicosis and hypothyroidism, respectively, is estimated to be 5%. AITD are significantly more common among women than among men, with a w/m ratio ranging from 5:1 to 10:1. Major specific antibodies in HT are TPO-Ab and Tg-Ab, but these antibodies also occur in ≈70% of GD patients. Similarly, the major antibody in GD thyroid hormone receptor antibody (stimulating TSHR-Ab) may also occur in a few patients with HT (blocking TSHR-Ab) [11].
HT is more common in iodine sufficient (e.g., USA) or excess (e.g., Japan) countries. In this condition, the thyroid gland is generally non-tender and firm, and is often enlarged with an irregular texture. Atrophy is often noted in the gland with diffusely infiltrated lymphocytes. The prevalence of HT is as high as 40% in elderly women. Nearly 50% of the patients diagnosed with TPO-Ab are euthyroid, while majority of the other patients have subclinical (mild) hypothyroidism (normal free T4 levels and elevated serum TSH) and only a small minority have severe hypothyroidism. The presence of TPO-Ab predicts the development of overt hypothyroidism at about 2.5% per year. Among the people with elevated TSH and positive TPO-Ab, about 4.5% per year develop overt hypothyroidism [11].
The Amsterdam AITD cohort is a 5-year follow-up study in a population at risk for AITD, namely in healthy women with one or more first- or second-degree relatives with proven AITD. The mean annual event rate was 1.5%, and the 5-year cumulative event rate was 7.5%. The incidence rate per 1000 women per year was 9.6 for overt hypothyroidism and 3.3 for overt hyperthyroidism [12].
It is unknown whether in CLT the goitrous (HT) and atrophic forms (primary myxedema) are variants of the same disease or different pathogenic entities. Conventional thyroid-related autoimmune parameters (Tg-Ab and ATPO-Ab) are unable to separate both variants/entities serologically. Other thyroid-specific cytotoxic antibodies, like TSH binding-inhibiting antibodies and TSH function-blocking antibodies, were determined. Analysis of cytotoxicity regarding thyroid size showed a high incidence of cytotoxic antibodies in atrophic disease (Tvol median 6 mL), where cytotoxic antibodies were detectable in 80% vs. 39% in goitrous disease (Tvol median 36 mL) [13].
Worldwide the most common thyroid disease entity is autoimmune hypothyroidism. Historically this is divided into primary atrophic (primary myxedema) and hypertrophic autoimmune hypothyroidism (CLT–HT). The primary atrophic variant was described in the late 1890s by Ord as “dependent on a destructive affection of the thyroid gland.” In 1912 Hashimoto described the hypertrophic variant later to be known as Hashimoto’s disease-thyroiditis (HT). No strict criteria exist to separate the atrophic and the goitrous form of hypothyroidism. Classification is often based on the presence or absence of goiter by clinical examination [14].
Ord’s and Hashimoto’s diseases have been reported to be distinct diseases, differing in human leukocyte antigen (HLA) types, the specific autoantibodies involved—blocking TSHR-Ab in the atrophic type of hypothyroidism, TPO-Ab and Tg-Ab in the hypertrophic form, and importance of humoral vs. cellular immunity for disease development [14].
Carlé et al., in a Danish population cohort of 247 patients with overt autoimmune hypothyroidism, measured thyroid volume (Tvol) by US. The aim was to verify or disprove whether Ord’s and Hashimoto’s are in fact two distinct disorders. Patients were divided in quartiles according to Tvol as follows:
Women (<6.7, 6.7–11.2, 11.3–17.4, >17.4 mL).
Men (<7.4, 7.4–12.3, 12.4–19.0, >19.0 mL).
Patients had smaller Tvol median compared with controls, 11.6 mL vs. 13.5 mL. Tvol, however, showed a Gaussian distribution in both women and men with no bimodal pattern. The patients with the smallest thyroid volumes (<6.7 resp. <7.4 mL) were biochemically more hypothyroid compared with others groups. Among other groups were found no statistically significant differences with regard to either serum TSH levels or serum T4 levels. With increasing Tvol, the levels of circulating antibodies (TPO-Ab, Tg-Ab) correspondingly increased and echostructure became more hypoechoic or even severely hypoechoic. No difference between groups was observed in prevalence of TSHR-Ab or duration of symptoms before hypothyroidism was diagnosed. In primary autoimmune hypothyroidism, Tvol follows a normal distribution. Cases with thyroid atrophy and goiter are only extremes within this distribution and do not represent separate disorders [14].
3.1.2 US Features of Hashimoto’s Thyroiditis
In 1983, French radiologist Espinasse described on US the diffused microechoic character of the thyroid parenchyma. Even though it is nonspecific, it appears thus a valuable sign in the diagnosis of chronic lymphocytic thyroiditis and Graves’ disease [15].
In the same year, Canadian radiologists drew attention to the importance of US in evaluating childhood thyroid disorders. In 25 patients with diffuse thyroid lesions (thyroiditis, Graves’ disease, euthyroid goiter, iodine-induced goiter, goitrous cretinism), US revealed only homogeneous thyroid enlargement or a nonspecific patchy echo pattern [16].
In 1989, German specialists Gutekunst et al. compared clinical, laboratory, and cytological findings with US patterns in 92 patients with CLT. A total of 29% had no clinical symptoms. According to laboratory values 13% of patients had undetectable antimicrosomal antibodies. The functional status according to TSH levels were ≈51% hypothyroid, ≈45% euthyroid and ≈4% hyperthyroid. Cytology alone was diagnostic in ≈91% patients. US revealed a scattered sonolucent echo in ≈ 95% patients, and in ≈49% a normal thyroid volume (women <18 mL, men <25 mL). Authors of this pioneering study concluded that US can suggest CLT. If antimicrosomal antibodies are undetectable or titres are not significant and/or clinical symptoms are uncertain, FNAB can confirm the US finding [17].
US is useful for measurement of thyroid size and assessing the echotexture in the HT—CLT patients. The US appearance may vary, which is likely due to the phase and severity of the disease process.
US imaging of classical HT pattern [1, 18]:

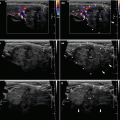
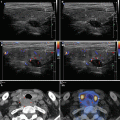
Fig. 3.1
(aa) A 30-year-old woman with Hashimoto’s thyroiditis (HT) of upper normal volume. Classic US appearance: inhomogeneous, mostly hypoechoic micronodular structure with thin hyperechoic fibrous septa; dorsally microlobulated margin; Tvol 16 mL, RL 8 mL, and LL 8 mL; transverse. (bb) Detail of RL with classic US appearance HT: inhomogeneous, mostly hypoechoic micronodular structure with thin hyperechoic fibrous septa; dorsally microlobulated margin; longitudinal. (cc) Detail of LL with classic US appearance HT: inhomogeneous, mostly hypoechoic micronodular structure with thin hyperechoic fibrous septa; dorsally microlobulated margin; longitudinal

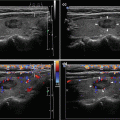
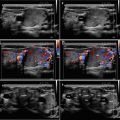
Fig. 3.2
(aa) A 41-year-old woman with Hashimoto’s thyroiditis (HT) of lower normal volume and fibrosis. US overall view: coarse structure; mixed echogenicity; hypoechoic micronodular structure with thick hyperechoic fibrous septa and areas; microlobulated margin; Tvol 9 mL, RL 4.5 mL, and LL 4.5 mL; transverse. (bb) Detail of RL with HT and fibrosis: coarse structure; mixed echogenicity; hypoechoic micronodular structure with thick hyperechoic fibrous septa and areas; microlobulated margin; longitudinal. (cc) Detail of LL with HT and fibrosis: coarse structure; mixed echogenicity; hypoechoic micronodular structure with thick hyperechoic fibrous septa and areas; microlobulated margin; longitudinal
The positive predictive value for micronodulation in diagnosing HT is 94.7% [19].
Caution! A diffusely heterogeneous, hypoechoic gland is not specific for HT and that may be seen in Graves’ disease, and subacute thyroiditis [8].
Thyroid volume changes throughout the course of Hashimoto’s disease. For the evaluation of goiter (Sect. 3.2), Gutekunst applied US criteria from the year 1988: Tvol >18 mL in women (Fig. 3.6), and Tvol >25 mL in men (Figs. 3.5 and 3.7) [20]. However, an exact cut-off is not determined for atrophic form (Sect. 3.3). The most frequently used value is 6 mL used in 1995 in a study, by Bogner, of atrophic (Figs. 3.11–3.14) and goitrous autoimmune thyroiditis [13].
In a study of 247 patients with overt autoimmune hypothyroidism, Carlé et al. measured Tvol by US, finding that 21% of the patients had goiter, 23% of women had thyroid volume >18 mL, and 12% of men had thyroid volume >25 mL [14].
Micronodules of HT can increase in size as focal lymphocytic thyroiditis (FLT) and be present as hypoechoic or hyperechoic nodules with ill-defined margins on US. The vascularity is markedly variable, without a distinguishing pattern. These so-called “pseudotumors” constituted 36% of the nodules of focal thyroiditis detected by US and are simulating nodular disease indistinguishable from thyroid cancer or lymphoma. The FLT may represent a milder or earlier presentation of the disease [8].
It is difficult to discriminate FLT from thyroid cancer if they both have suspicious US findings (see Chap. 15). Tg-Ab positivity, presence of a HT pattern (diffusely heterogeneous hypoechogenicity) on US, and absence of calcification in nodules were associated with FLT (specificity of 99% and positive predictive value of 96%, but sensitivity only 45%). However, 20% of FLTs had calcification, which could indicate malignancy. Therefore, absence or presence of calcification in nodules showed relatively low predictive value for FLT. In conclusion, suspicious thyroid nodules with benign cytologic results on initial FNAB could be followed-up only by US, if there is seropositivity of Tg-Ab, no calcifications in nodules, and presence of a HT pattern on US [21].
The US features and vascularity of nodular HT are extremely variable. In a cohort of 64 patients with HT there occurred a solitary nodule (Figs. 8.5aa and 8.6aa) in 36% and the setting of five or more nodules (Fig. 3.8aa) in 23% of cases. Fifty-five percent of cases of nodular HT occurred within a US background of diffuse HT, and 45% of cases occurred within a US normal thyroid parenchyma. The mean diameter of nodule was 15 ± 7.33 mm. Nodules were most commonly solid in 69% and hypoechoic in 47%. Twenty percent of nodules had calcifications (nonspecific bright reflectors, macrocalcifications, or “eggshell” – Fig. 9.9), and 5% of nodules had colloid cavity (Fig. 3.8aa). The margins were well-defined in 60% and ill-defined in 40% of nodules, and 27% had a hypoechoic halo (Fig. 8.6aa). On CFDS analysis, 35% of nodules were hypervascular, 42% isovascular or hypovascular (Fig. 8.6bb), and 23% avascular [22].
Currently high-resolution sonography instruments are a useful diagnostic tool for the evaluation of diffuse thyroid disease such as HT. According to Kim et al., a combination of ≥3 US features of HT has a high sensitivity and specificity for the identification of HT compared with the use of ≤2 US features. No visualization of US features related to HT on real-time thyroid sonography can rule out the existence of asymptomatic HT [23].
The presence of perithyroidal lymph nodes (LN) is useful in diagnosis of the HT when correlated with US, clinical, and laboratory findings. However, it should be kept in mind that these LN may also correspond to underlying malignant processes, such as PTC and lymphoma. In doubtful cases, FNAB may be required to differentiate between benign (reactionary/inflammatory origin) and malignant LN [18].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree