Heart
The imaging evaluation of the infant or child with suspected heart disease begins with a chest radiograph followed by transthoracic echocardiography (1). In many lesions, echocardiography can accurately assess both anatomic and functional abnormalities and occasionally can obviate cardiac catheterization. Morphologic details of the valves, outflow tracts, relative size of the ventricles, and septation defects are particularly well demonstrated by echocardiography. If echocardiography is diagnostic, the patient may go directly to surgery without additional imaging. However, in some patients, access to an optimal acoustic window can be limited by body habitus, hyperinflation of the lungs, or postoperative wires and prosthetic valves. In these instances, magnetic resonance imaging (MRI) and computed tomography (CT) may add useful information. MRI and CT are used in the diagnosis and postoperative assessment of extracardiac and complex intracardiac lesions, the pulmonary artery confluence, coronary artery ostia, and/or systemic collateral arteries (2,3,4,5,6,7,8,9). MRI is also sometimes used to provide functional data useful in evaluating physiology.
Based on the presence or absence of clinical cyanosis and the extent of pulmonary blood flow demonstrated on chest radiography, congenital heart disease (CHD) is classified as cyanotic heart disease with decreased vascularity, cyanotic heart disease with increased vascularity, acyanotic heart disease with increased vascularity, acyanotic heart disease with normal vascularity, and pulmonary venous hypertension. This format will be followed for discussion of congenital heart disease in this chapter. The plain radiographic findings and CT and/or MR findings of the most common congenital heart diseases and some acquired lesions will be discussed and illustrated.
The differential diagnosis of CHD is based on presence or absence of clinical cyanosis and the amount of pulmonary blood flow.
CYANOTIC HEART DISEASE WITH DECREASED VASCULARITY
Cyanosis occurs when more than 5 g/dL of reduced hemoglobin is present in capillary blood. In central cyanosis due to congenital heart disease, a portion of the systemic venous blood reaches the aorta without passing through the lungs. This can occur as a result of a right heart obstruction with right to left shunting through a septation defect (discussed in this section) or a primary admixture lesion (discussed in the following section).
Most patients with cyanosis due to congenital right heart obstruction present on the first day of life. However, if there is mild obstruction of the outflow tract or if adequate systemic collateral vessels have formed, some patients will present later in life.
Most patients with cyanosis due to congenital right heart obstruction present on the first day of life.
TETRALOGY OF FALLOT
Tetralogy of Fallot accounts for about 10% of congenital heart defects and is the most common congenital heart defect causing cyanosis. The classic components of tetralogy of Fallot are right ventricular subpulmonic obstruction, a perimembranous (subaortic) ventricular septal defect (VSD), overriding of the aorta, and right ventricular hypertrophy. Other anomalies include peripheral pulmonary arterial stenosis and anomalous origin of the left anterior descending artery from the right coronary artery. A right aortic arch is present in approximately 25% of patients.
Tetralogy of Fallot is the most common congenital heart defect causing cyanosis.
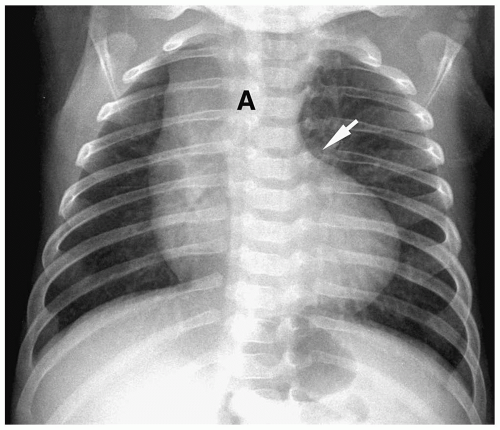
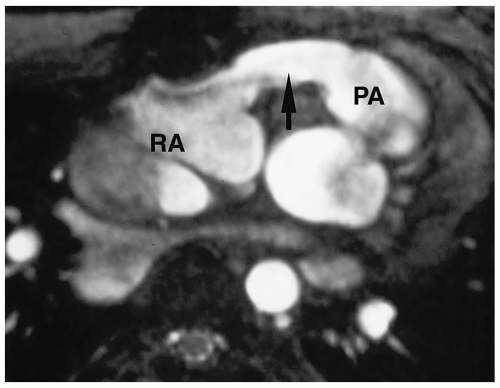
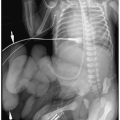
Chest radiographic findings include normal cardiac size, uplifting of the cardiac apex secondary to right ventricular hypertrophy, decreased pulmonary vascularity, and a small or concave main pulmonary artery (Fig. 4.1). MRI and CT have been used to demonstrate the VSD and the size and confluence of the central pulmonary arteries, which is critical information for planning palliative repair and definitive surgical correction (Fig. 4.2).
Radiographic findings in tetralogy of Fallot include normal cardiac size, uplifted cardiac apex, decreased vascularity, and a small main pulmonary artery.
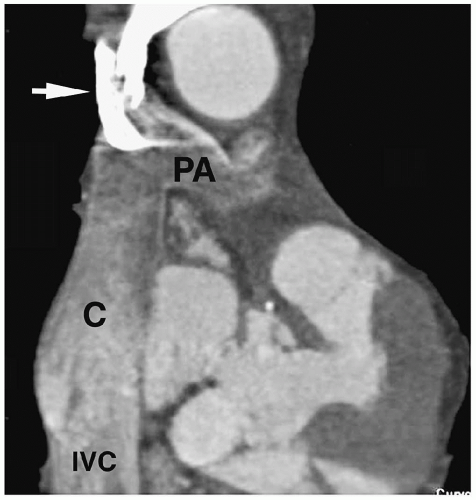
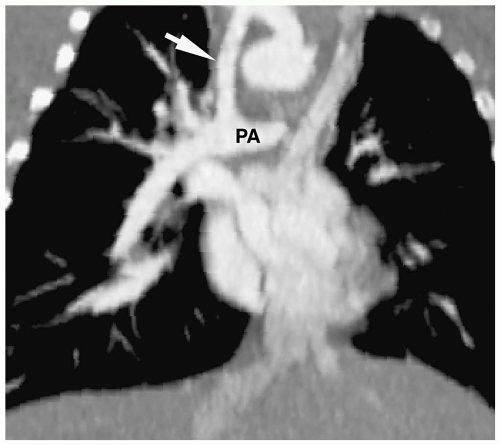 Figure 4.3 Blalock-Taussig anastomosis. Coronal reformatted CT angiogram shows a patent Blalock-Taussig shunt (arrow) between the right subclavian artery and right pulmonary artery (PA). |
Patients initially are treated with palliative surgery, which is performed using a Blalock-Taussig anastomosis (subclavian artery to pulmonary artery) (Fig. 4.3). Definitive repair involves closing the septal defect and relieving the right ventricular outflow obstruction by placing a patch across either the outflow tract or the pulmonary valve. Postoperative complications include aneurysmal dilatation of the outflow patch (Fig. 4.4), right ventricular
outflow tract insufficiency, and residual VSD. These sequelae may lead to volume overload and predispose to right ventricular failure and arrhythmias.
outflow tract insufficiency, and residual VSD. These sequelae may lead to volume overload and predispose to right ventricular failure and arrhythmias.
Patients with tetralogy are initially treated with palliative surgery using a Blalock-Taussig anastomosis.
ABSENT PULMONARY VALVE
This condition has some findings similar to those seen in tetralogy of Fallot, including a VSD, overriding of the aorta, and narrowing of the pulmonary outflow tract. However, the pulmonary valve leaflets are rudimentary, resulting in severe pulmonary insufficiency. The pulmonary arteries are characteristically enlarged (rather than small as in Fallot tetralogy), and they may compress the trachea and bronchi, leading to respiratory distress (Fig. 4.5). Surgical repair is essentially similar to that of tetralogy of Fallot.
TRICUSPID ATRESIA
Tricuspid atresia accounts for about 1% of all congenital heart disease. In tricuspid atresia, the tricuspid valve and the inflow portion of the right ventricle are absent. The right ventricle
is usually small. An associated VSD is common. Survival depends on the presence of the VSD or on an interatrial communication, either an atrial septal defect (ASD) or patent foramen ovale, or on the ductus remaining open early in life, in order for blood to reach the lungs. The left heart is normal. The great vessels are normally positioned in 70% of patients and have dextro-transposition in 30% of patients. A right-sided aortic arch occurs in 5% to 10% of patients.
is usually small. An associated VSD is common. Survival depends on the presence of the VSD or on an interatrial communication, either an atrial septal defect (ASD) or patent foramen ovale, or on the ductus remaining open early in life, in order for blood to reach the lungs. The left heart is normal. The great vessels are normally positioned in 70% of patients and have dextro-transposition in 30% of patients. A right-sided aortic arch occurs in 5% to 10% of patients.
In tricuspid atresia, the tricuspid valve and the inflow portion of the right ventricle are absent.
Chest radiography usually shows a normal heart size, decreased pulmonary vascularity, and a concave main pulmonary artery segment (Fig. 4.6). If there is a large VSD, the heart may be enlarged, and the pulmonary vascularity may be normal or increased.
Radiographic findings in tricuspid atresia are a normal heart size, decreased vascularity, and a concave main pulmonary artery segment.
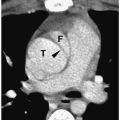
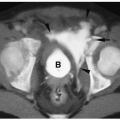
MR and CT can delineate the presence of an ASD or VSD, the size of the right ventricle, and the relationship of the great vessels. A characteristic finding of tricuspid atresia on MR and CT is increased fat deposition in the right atrioventricular groove (Fig. 4.7).
Patients with tricuspid atresia have undergone a number of different operations over the past two decades. The early surgery was a Glenn shunt—an anastomosis between the superior vena cava and the right pulmonary artery (Fig. 4.8). This shunt resulted in isolation of the right lung from hepatic blood flow and led to the development of pulmonary arteriovenous fistulas, and it is no longer performed.
The subsequent surgical repair was a Fontan procedure, which used a valved conduit between the right atrium and the left pulmonary artery (Fig. 4.9). More recent modifications include anastomosis of the inferior vena cava to pulmonary artery anastomosis (extracardiac
Fontan) and anastomosis of the inferior vena cava and superior vena cava to each other and then to the pulmonary artery (total cavopulmonary Fontan) (Fig. 4.10). Complications intrinsic to all Fontan procedures include narrowing or leaks in the Fontan graft, ventricular outflow obstruction, and right atrial enlargement.
Fontan) and anastomosis of the inferior vena cava and superior vena cava to each other and then to the pulmonary artery (total cavopulmonary Fontan) (Fig. 4.10). Complications intrinsic to all Fontan procedures include narrowing or leaks in the Fontan graft, ventricular outflow obstruction, and right atrial enlargement.
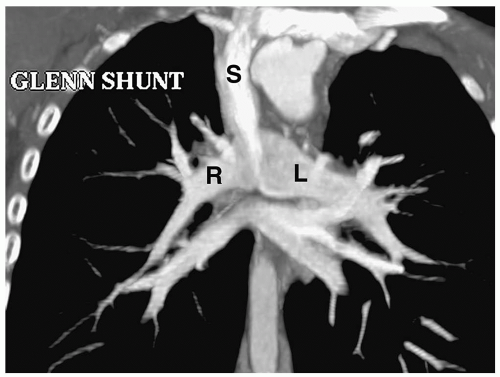 Figure 4.8 Glenn shunt. Coronal reconstructed CT image shows anastomosis of the superior vena cava (S) with the right pulmonary artery, (R). (L), pulmonary artery. |
PULMONARY ATRESIA WITH INTACT SEPTUM
In pulmonary atresia with intact ventricular septum, continuity between the right ventricle and pulmonary arteries is absent. The pulmonary valve is atretic, and there is hypoplasia or atresia of the main pulmonary artery. The right and left pulmonary arteries may or may not connect with each other. Lack of connection is termed nonconfluence. The right ventricle is small, and the walls may be hypertrophied. Because there is no associated VSD, blood is diverted from the right atrium to the left atrium via the foramen ovale. Survival depends on the presence of a patent ductus arteriosus (PDA) or systemic bronchial and/or intercostal arteries. A right-sided arch is present in 30% of patients.
In pulmonary atresia with intact ventricular septum, continuity between the right ventricle and pulmonary arteries is absent.
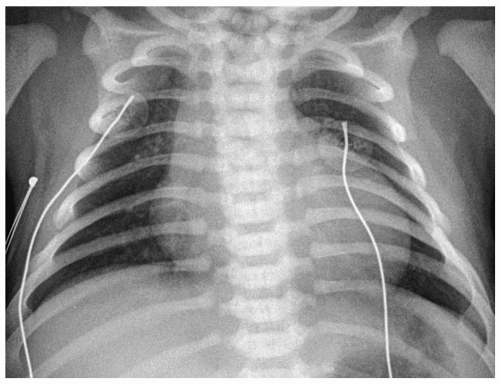 Figure 4.11 Pulmonary atresia with intact septum. Frontal radiograph of a newborn with cyanosis shows cardiac enlargement and diminished pulmonary vascularity. |
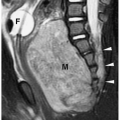
Chest radiographs typically show right heart enlargement, an upturned ventricular apex, marked concavity in the region of the main pulmonary artery, and decreased pulmonary vascularity (Fig. 4.11). The left ventricle, which incurs most of the volume load, eventually enlarges. The aorta is usually dilated. MRI and CT have been used to demonstrate the size and confluence or nonconfluence of the pulmonary arteries, the presence of aortic-pulmonary collaterals, and the presence of a VSD (Fig. 4.12).
Treatment is surgery to establish flow to the pulmonary artery.
EBSTEIN ANOMALY
Ebstein anomaly is characterized by displacement of the septal and posterior tricuspid valve leaflets into the right ventricle and adherence of the anterior valve leaflet to the tricuspid valve annulus. As a result, the right atrium projects into the right ventricle, which is described as atrialized. The right ventricle is small and slow to empty. A right-to-left shunt through a patent foramen ovale or ASD is virtually always present.
Ebstein anomaly is characterized by displacement of tricuspid valve leaflets into the right ventricle and adherence of the anterior valve leaflet to the tricuspid valve annulus.
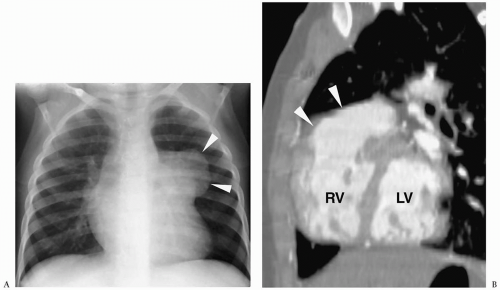
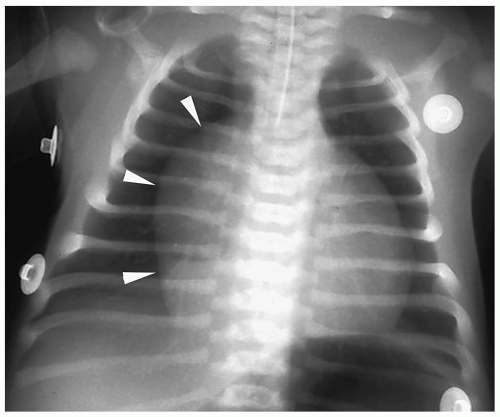 Figure 4.13 Ebstein anomaly. Frontal radiograph demonstrates markedly decreased pulmonary vascularity and cardiomegaly with a prominent right heart (arrowheads). |
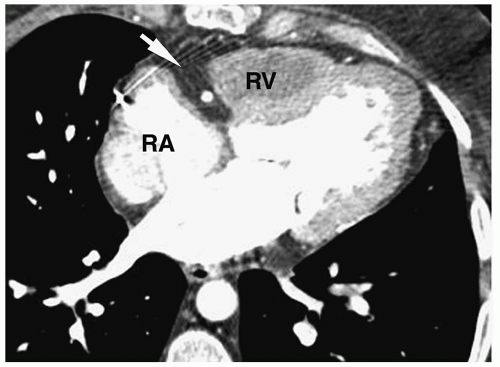
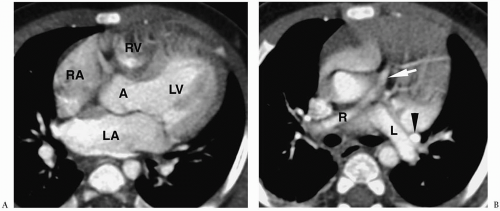
Radiographic findings include marked cardiomegaly due to right atrial enlargement, decreased pulmonary vascularity, and a concave pulmonary artery segment (Fig. 4.13). The aortic arch is nearly always left-sided. MRI and CT can directly demonstrate the three chambers in the right heart (right atrium, atrialized superior right ventricle, and small inferior right ventricle) (Fig. 4.14).
Radiographic findings of Ebstein anomaly include marked cardiomegaly and decreased pulmonary vascularity.
Patients with severe Ebstein anomaly are treated with reduction and plication of the right atrial free wall and tricuspid valve reconstruction. Patients with milder malformations may need no interevention. Palliative surgery has no role in treatment.
CYANOTIC HEART DISEASE WITH INCREASED VASCULARITY
In these lesions, cyanosis results from incomplete separation of the right and left circulations, resulting in admixture of deoxygenated systemic venous blood and oxygenated pulmonary venous blood. The vascularity is generally increased, but it needs to be recognized that it can be normal or decreased if there is coexistent pulmonary stenosis.
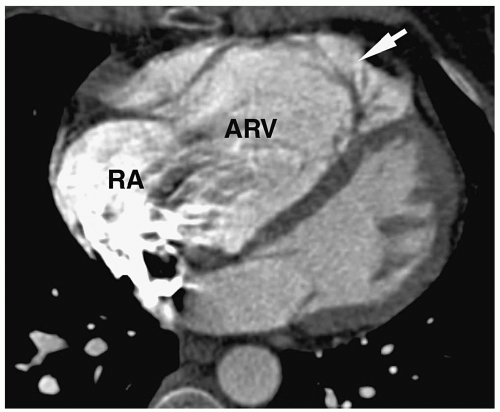 Figure 4.14 Ebstein anomaly. CT in a young adult shows a contrast-filled right atrium (RA), large atrialized superior right ventricle (ARV), and small inferior ventricle (arrow). |
DEXTRO-TRANSPOSITION OF THE GREAT VESSELS
Dextro-transposition of the great vessels (D-TGV), also termed complete transposition of the great vessels, accounts for about 8% of congenital cardiac malformations and is the most common congenital heart lesion causing cyanosis on the first day of life. In D-TGV, the pulmonary artery arises from the morphologic left ventricle, and the aorta and coronary arteries arise from the morphologic systemic right ventricle (i.e., ventricular arterial discordance). The ascending aorta is positioned to the right and anterior to the main pulmonary artery. The pulmonary and systemic circulations exist in parallel rather than in series, and survival depends on bidirectional shunting between the two sides of the heart. Connections between the two circulations include VSD (40% of patients), ASD, PDA, and patent foramen ovale.
D-TGV is characterized by ventricular arterial discordance.
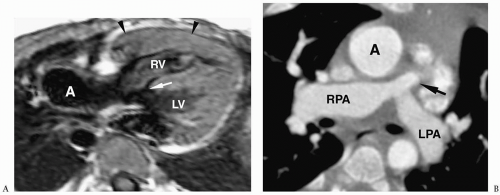
The classic radiographic findings include a narrowed superior mediastinum due to abnormal position of the great vessels and a small thymus, a concave main pulmonary artery segment, mild to moderate cardiomegaly, and increased pulmonary vascularity (Fig. 4.15). The radiographic appearance of the cardiac silhouette has been termed egg on a string. Of note, these classic findings are present in only about 50% of patients. In the remaining patients, the cardiac size can be normal and the vascularity can be normal or decreased in the first few days of life. MRI and CT have been used to demonstrate the right ventricular origin of the aorta and coronary ostia, the left ventricular origin of the pulmonary artery, and the presence or absence of a shunt (Fig. 4.16).
Radiographic findings of D-TGV include a narrowed superior mediastinum, cardiomegaly, and increased vascularity.
Before the advent of the arterial switch or Jatene operation, patients with D-TGV underwent correction at the atrial level. The two techniques used to repair D-TGV were the Mustard technique, which used pericardial tissue in the reparative baffle, and the Senning technique, which was based on infolding of the atrial walls (Fig. 4.17). Patients who had undergone repair at the atrial level often developed baffle complications in the superior limb of the baffle, such as stenosis and leak, which led to recurrent cyanosis and right heart failure. The arterial switch is now the procedure of choice for surgical repair of D-TGV. In this procedure, the great arteries are transected above their valves and reanastomosed to the opposite great vessels (Fig. 4.18). The coronary arteries are also switched.
The arterial switch is the procedure of choice for surgical repair of D-TGV.
Variants of Transpostion
Levo-transposition of the Great Arteries.
Levo-transposition of the great vessels (L-TGV) is characterized by inversion of the ventricles and atrioventricular valves. The pulmonary and systemic circuits are in series and not in parallel as in D-TGV. Blood flows from the right atrium into the right-sided, morphologic left ventricle and then into the main pulmonary artery. Oxygenated blood returns from the lungs via the pulmonary veins to the left atrium and then into the aorta via the left-sided, morphologic right ventricle. The ascending aorta
is positioned to the left and anterior to the main pulmonary artery (Fig. 4.19). Patients are asymptomatic unless there are coexisting lesions. In many cases (75%), an associated VSD is present. Other common heart defects include pulmonary stenosis, either valvular or subvalvular, and coarctation. The heart is often normal in size and the vascularity may be increased, decreased, or normal depending on the presence or absence of a VSD or pulmonary stenosis.
is positioned to the left and anterior to the main pulmonary artery (Fig. 4.19). Patients are asymptomatic unless there are coexisting lesions. In many cases (75%), an associated VSD is present. Other common heart defects include pulmonary stenosis, either valvular or subvalvular, and coarctation. The heart is often normal in size and the vascularity may be increased, decreased, or normal depending on the presence or absence of a VSD or pulmonary stenosis.
L-TGV is characterized by inversion of the ventricles and atrioventricular valves.
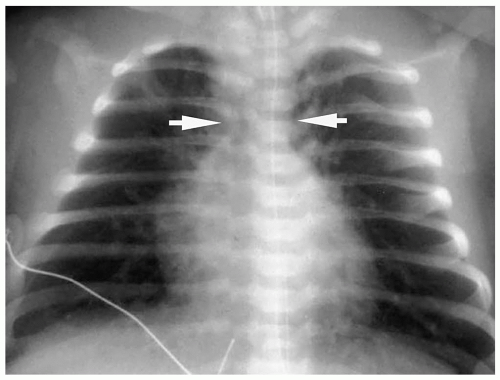 Figure 4.15 Dextro-transposition of the great vessels (D-TGV). Chest radiograph in a newborn with cyanosis shows a narrow superior mediastinum (arrows), mild prominence of the right heart border, and increased pulmonary vessels.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|