Neck
Plain radiographs remain the imaging study of choice to evaluate acute airway obstruction and bony abnormalities. Ultrasonography is usually the initial imaging method for evaluating suspected neck masses and for differentiating cystic and solid lesions, and it is also the study of choice to evaluate vascular lesions (1). Computed tomography (CT) or magnetic resonance imaging (MRI) are used after sonography if further determination of the precise origin of a mass lesion, its extent, and its effect on adjacent structures is needed for clinical management (2,3).
Plain radiographs remain the imaging study of choice to evaluate acute airway obstruction.
Ultrasonography is usually the imaging method for evaluating suspected neck masses.
NORMAL ANATOMY
RETROPHARYNGEAL SOFT TISSUES
The width of the retropharyngeal space is usually equal to one half of the width of the vertebral body above the level of the fourth cervical vertebral body (C4). Below this level, the soft tissue space may normally be wider, usually about the width of the vertebral body. A thicker prevertebral space usually indicates pathology, especially if the neck is extended and the pharyngeal airway is distended. If the neck is flexed or the airway is collapsed, usually because the infant is crying, the prevertebral tissues become more prominent and may mimic a mass (Fig. 1.1). If radiographs are technically suboptimal or equivocal, fluoroscopy can be helpful in confirming the presence or absence of a mass. If the diagnosis is still equivocal, CT can be performed.
If the neck is flexed or the airway is collapsed, the prevertebral tissues become more prominent and may mimic a mass.
LYMPH NODES
Lymph nodes are not routinely seen in infants and young children at imaging. Occasionally, small nodes are observed in older children and adolescents. These nodes can be considered normal if they are 5 mm or less in longest diameter. Normal lymph nodes are hypoechoic with an echogenic linear hilum on sonography, soft tissue attenuation on CT, low signal intensity on T1-weighted images, and high signal intensity on T2-weighted and fat-suppressed images. The hilar region is vascular on color Doppler imaging.
Lymph nodes are not routinely seen in infants and young children at imaging.
VASCULAR VARIANTS
The most common variation of normal anatomy is asymmetry in the size of the internal jugular veins. It is not unusual for the right internal jugular vein to be larger than the left, presumably due to predominance of the right cerebral venous drainage.
THYROID GLAND
The normal thyroid gland is composed of two lateral lobes, which are joined by an isthmus (4). Occasionally an extra lobe, the pyramidal lobe, extends superiorly from the isthmus in the midline. The gland is bordered posterolaterally by the longus colli muscle, common carotid artery, and internal jugular vein, medially by the trachea on the right and esophagus on the left, and anterolaterally by the sternocleidomastoid and strap muscles.
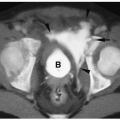
The normal thyroid gland is hyperechoic on sonography and hyperintense on CT.
The normal thyroid gland is a soft tissue structure that is hyperechoic on sonography and hyperintense on CT relative to surrounding muscle (Fig. 1.2). It enhances intensely because of its extensive vascular supply. Thyroid size and weight vary with patient age. In infants and young children, the lateral lobes measure between 1 and 1.5 cm in transverse diameter, 2- to 3-cm vertically, and 0.2 to 1.2 cm in anteroposterior diameter. In adolescents and adults, the lateral lobes measure 2 to 4 cm in transverse dimension, 5- to 8-cm vertically, and 1 to 2.5 cm in anteroposterior diameter. The right lobe usually is larger than the left lobe.
Laryngeal skeleton ossification increases with increasing patient age.
Laryngeal skeleton ossification increases with increasing patient age. Thyroid cartilage calcification may be evident by the end of the first decade, and the pattern of calcification usually is irregular and asymmetric. Well-defined or extensive thyroid calcification is unusual prior to 13 years of age and should suggest cardiovascular disease, skeletal dysplasia,
or hypercalcemic states. Ossification of the cricoid and hyoid cartilages also begins in late childhood or adolescence.
or hypercalcemic states. Ossification of the cricoid and hyoid cartilages also begins in late childhood or adolescence.
PARATHYROID GLAND
There are usually four parathyroid glands. The paired superior glands have a fairly constant position near the upper surface of the thyroid lobes. The inferior parathyroid glands are found in close proximity to the lower pole of the thyroid gland. Similar to the thyroid gland, the migration of the parathyroid glands may be arrested or the glands may migrate below the level of the thyroid gland into the mediastinum. Fewer than 5% are found in ectopic locations in the neck or in a substernal position. Occasionally, there may be more than four parathyroid glands. These supernumerary glands are frequently found in the mediastinum in association with the thymus. The normal parathyroid glands are difficult to visualize by imaging because of their small size.
CONGENITAL NECK MASSES
Approximately 85% of masses are benign, and most are located in the infrahyoid portion of the neck. Congenital cystic masses (cystic hygromas, hemangiomas, branchial cleft cysts, thyroglossal duct cysts, teratomas, dermoids) and lymphadenopathy account for nearly all benign neck lesions (5,6,7,8,9,10). Primary malignant tumors in children are usually lymphoma or rhabdomyosarcoma.
Most neck masses are benign and are located in the infrahyoid portion of the neck.
CYSTIC HYGROMAS
Cystic hygromas, also known as lymphangiomas, result from congenital blockage of lymphatic drainage. Approximately 50% of cystic hygromas are discovered at birth, and 90% are diagnosed before the end of the second year. About 75% are found in the neck, with about 3% to 10% of these extending into the mediastinum. Twenty percent are found in the
axilla. Rare isolated lesions have also been described in the mediastinum, retroperitoneum, bone, and abdominal viscera.
axilla. Rare isolated lesions have also been described in the mediastinum, retroperitoneum, bone, and abdominal viscera.
Cystic hygromas result from congenital blockage of lymphatic drainage.
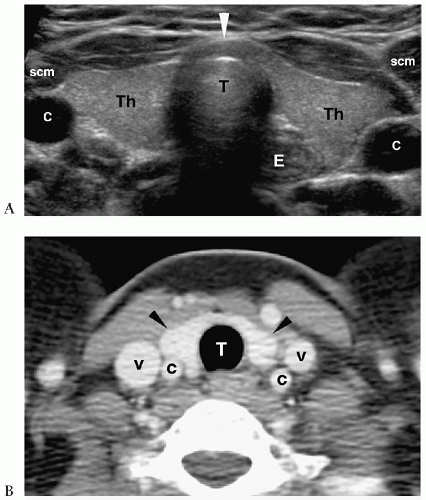
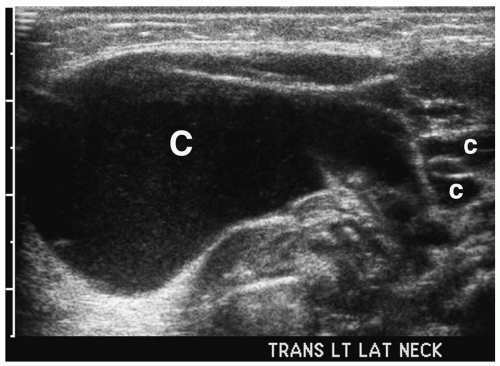 Figure 1.3 Cystic hygroma in an 11-day-old girl with a left-sided neck mass. Transverse sonogram of the neck shows a large simple cyst (C) with several smaller adjacent cysts (c). |
Cervical cystic hygromas present as painless, compressible masses in the posterior triangle of the neck. They typically infiltrate both anteriorly and posteriorly and as a result, they may cause airway and esophageal compression, leading to dyspnea and dysphagia. Although usually discovered in an otherwise healthy infant, cystic hygromas can be associated with Noonan syndrome, Turner syndrome, fetal alcohol syndrome, Robert syndrome, familial pterygium colli, and several trisomy syndromes.
Cystic hygroma presents as an asymptomatic mass in the posterior cervical triangle.
On sonography, CT, and MRI, cystic hygroma appears as a thin-walled, multilocular mass (11,12) (Fig. 1.3). The lesion is predominantly fluid filled, although it often contains solid foci. Similar to other cystic lesions, the echogenicity, attenuation value, or signal intensity can increase secondary to hemorrhage, infection, or high lipid content (Fig. 1.4). The cyst walls also become thicker with infection. Infiltration into adjacent soft tissues
is common with larger lesions. The typical location in the posterior neck, multicystic appearance, and age of the patient usually allow differentiation of cystic hygroma from other cervical lesions.
is common with larger lesions. The typical location in the posterior neck, multicystic appearance, and age of the patient usually allow differentiation of cystic hygroma from other cervical lesions.
Cystic hygroma appears as a thin-walled, multilocular mass that is predominantly fluid filled.
HEMANGIOMAS
Hemangiomas are congenital vascular abnormalities. Most are clinically evident before patients are 6 months of age. They typically present as soft cutaneous or subcutaneous masses with bluish discoloration. Hemangiomas often undergo a period of initial growth before spontaneously involuting in early childhood. Deep subcutaneous hemangiomas regress more slowly and less completely than superficial lesions.
Hemangiomas are congenital vascular abnormalities clinically evident before 6 months of age.
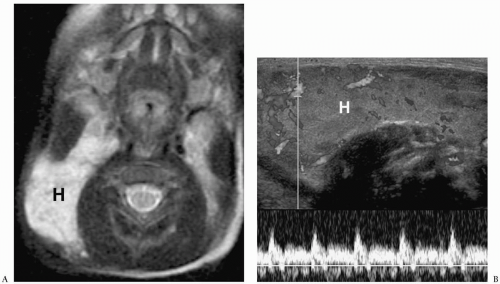
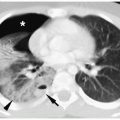
Hemangiomas appear hypoechoic on sonography, hypoattenuating on CT, hypointense on T1-weighted images, and hyperintense on T2-weighted images (Fig. 1.5). Occasionally, they contain punctate calcifications, representing phleboliths. Hypervascularity can be documented on pulsed Doppler imaging. Hemangiomas may opacify homogeneously or they may show peripheral enhancement with gradual central enhancement after administration of iodinated contrast material or gadolinium.
Hypervascularity can be documented on imaging.
BRANCHIAL CLEFT CYSTS
Approximately 90% of branchial abnormalities arise from the second branchial cleft, 8% from the first branchial cleft, and the remainder from the third branchial cleft. Branchial cysts, sinuses, or fistulas develop when there is failure of obliteration of the branchial clefts or pouches. A branchial sinus opens externally to the skin, and a fistula communicates externally to the skin and internally to the pharynx. A cyst has no internal or external openings. Branchial cleft cysts are the most common of the branchial apparatus abnormalities.
Approximately 90% of branchial abnormalities arise from the second branchial cleft.
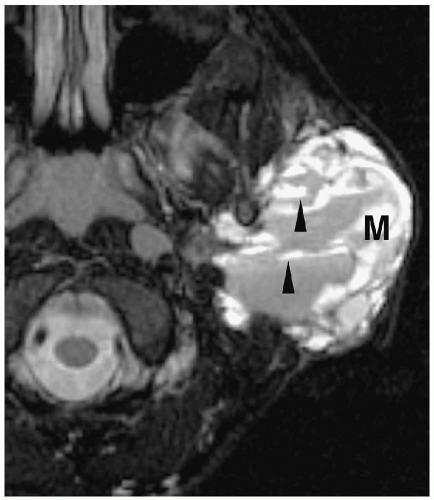
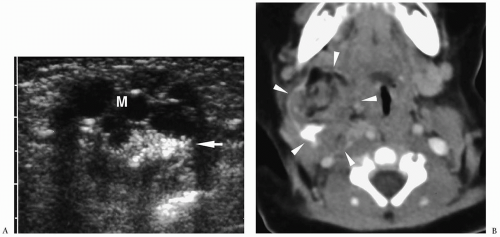
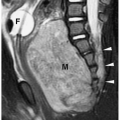
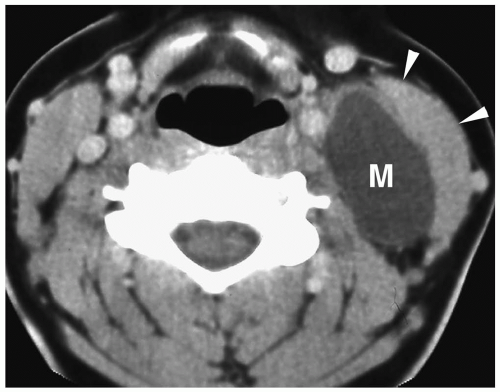 Figure 1.6 Second branchial cleft cyst in a young male. Contrast-enhanced CT shows an ovoid mass (M) of water density posterior to the left sternocleidomastoid muscle (arrowheads). |
They typically present as masses in the upper neck along the anterior border of the sternocleidomastoid muscle and are usually found in patients between 10 and 40 years of age. These cysts do not communicate with either the skin or the pharynx and hence do not drain. Infected cysts may be tender and painful. Approximately 5% of cysts are bilateral.
Branchial cleft cysts appear as fluid-filled masses in the lateral neck anterior to the sternocleidomastoid muscle.
On sonography, CT, and MRI, branchial cleft cysts appear as sharply marginated, thinwalled, fluid-filled masses in the lateral neck (Fig. 1.6) (1,2,3,4,5,6,7,8,9,10). Most cysts lie anterior to the sternocleidomastoid muscle, but rarely they may be found posterior to the sternocleidomastoid muscle, in the parapharyngeal space, or within the substance of the parotid gland. They are hypoechoic on sonography, low attenuation on CT, hypointense on T1-weighted MR images, and hyperintense on T2-weighted images. With infection, the wall of the cyst becomes irregular, thick, and enhances; the echogenicity, density, and signal intensity of the fluid increases; and the adjacent tissues become edematous and poorly defined (Fig. 1.7). In these instances, the cyst may mimic abscess or lymphadenopathy. The upper-lateral neck location usually helps differentiate branchial cleft cysts from the other anterior triangle lesions.
THYROGLOSSAL DUCT CYSTS
Thyroglossal duct cysts arise from remnants of the embryonic thyroglossal duct that connects the foramen cecum at the base of the tongue to the thyroid gland. Approximately 65% of thyroglossal duct cysts are located below the level of the hyoid bone, 20% are suprahyoid,
and 15% are at the level of the hyoid bone. Most thyroglossal duct cysts present during the first decade of life as midline or slightly off-midline masses in the anterior part of the neck. Characteristically, they move with swallowing.
and 15% are at the level of the hyoid bone. Most thyroglossal duct cysts present during the first decade of life as midline or slightly off-midline masses in the anterior part of the neck. Characteristically, they move with swallowing.
Approximately 65% of thyroglossal duct cysts are located below the level of the hyoid bone.
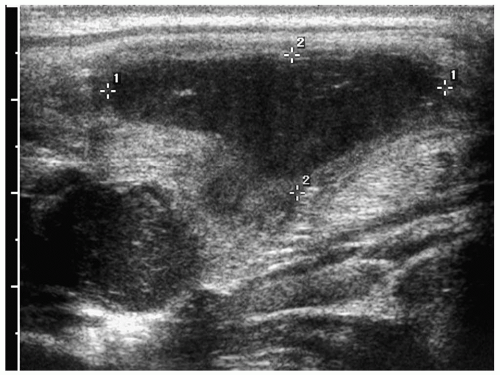 Figure 1.7 Infected branchial cleft cyst. Oblique sonogram of the neck in a 17-year-old boy with neck swelling shows a complex fluid collection (cursors) with echogenic mobile internal contents. |
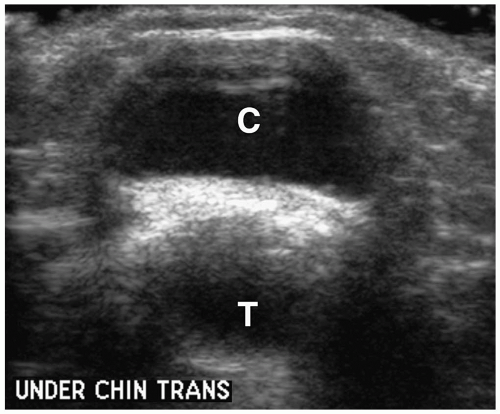 Figure 1.8 Thyroglossal duct cyst in an 8-year-old girl. Transverse sonogram beneath the chin shows a midline cystic mass (C) just anterior to the trachea (T). |
On imaging, uncomplicated cysts have well-defined walls and water contents (Fig. 1.8) (1,2,3,4,5,6,7,8,9,10,13). The internal echogenicity, attenuation, or signal intensity increases if the cyst contains highly proteinaceous contents, blood, or purulent material. Thick walls and thin internal septa also can be identified. Suprahyoid cysts are usually midline in location, whereas cysts below the hyoid bone tend to have midline and off-midline components. Extension into the hyoid bone may occur. The midline location of thyroglossal duct cysts helps to differentiate them from branchial cleft cysts. Imaging studies should be closely scrutinized and the appearance of the thyroid documented to make sure that a midline mass is not the only functioning thyroid tissue prior to surgical exploration.
The midline location of thyroglossal duct cysts helps to differentiate them from branchial cleft cysts and cystic hygromas.
DERMOID CYSTS AND TERATOMAS
Dermoid cysts are composed of two germ-cell layers (ectoderm and mesoderm), whereas teratomas contain elements from all three germ-cell layers. Cervical lesions are usually large, bulky masses that are clearly recognizable at birth. Large lesions may cause stridor, dyspnea, or dysphagia. Most dermoid cysts and teratomas arise in the anterior suprahyoid neck (submental area). They may be midline or off-midline in location and adjacent to or within a thyroid lobe (14). Demonstration of a mass containing fluid and fat or calcifications should suggest the diagnosis of dermoid or teratoma (Fig. 1.9). Teratomas and dermoids have similar appearances, and a specific diagnosis requires pathologic examination.
Dermoid cysts and teratomas may be midline or off-midline in location.
Demonstration of fluid and fat or calcifications should suggest the diagnosis of dermoid or teratoma.
CERVICAL THYMIC CYSTS
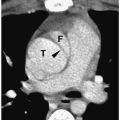
Cervical thymic cysts develop from vestiges of the thymopharyngeal ducts or from areas of cystic degeneration of the thymus gland. About two thirds of cysts are diagnosed in the first decade of life, with the remaining cases being diagnosed in the second and third decades. Most present as slowly enlarging masses. They can occur anywhere in the neck from the angle of the mandible to the sternum (15). The imaging appearance is similar to that of cysts elsewhere in the body (Fig. 1.10). The continuity with normal thymic tissue allows differentiation of thymic cysts from other cystic masses.
Continuity with normal thymic tissue allows differentiation of cervical thymic cysts from other cystic masses.
RANULAS
A ranula is a mucus retention cyst that arises from an obstructed sublingual or minor salivary duct in the floor of the oral cavity. Causes of obstruction include postinflammatory stricture, trauma, or calculus. There are two types of ranulas: simple and plunging. The more common simple ranula is restricted to the sublingual space, usually presenting as a
painless, bluish, intraoral mass beneath the tongue. The plunging or deep ranula extends beyond the sublingual space through the mylohyoid muscle. It presents as a painless submandibular or submental neck mass with or without an associated mass in the floor of the mouth.
painless, bluish, intraoral mass beneath the tongue. The plunging or deep ranula extends beyond the sublingual space through the mylohyoid muscle. It presents as a painless submandibular or submental neck mass with or without an associated mass in the floor of the mouth.
The more common simple ranula is restricted to the sublingual space.
The plunging or deep ranula presents as a submandibular or submental neck mass.
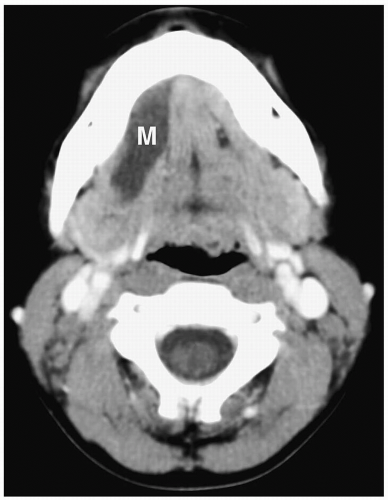 Figure 1.11 Simple ranula in an 18-year-old girl. Contrast-enhanced CT scan shows a thin-walled, low-attenuation mass (M) in the floor of the mouth. |
On sonography, CT, and MRI, simple ranulas appear as well-defined, thin-walled lesions above the mylohyoid muscle in the floor of the mouth (Fig. 1.11). Plunging ranulas may have more irregular contours, infiltrate soft tissues, and cross the midline. Most ranulas demonstrate homogeneous, near-water contents. The lesions may have increased echogenicity, attenuation, or signal intensity and a thick wall if they become infected (Fig. 1.12). Imaging features of dermoid cysts and ranulas may overlap. The location of this lesion in the floor of the mouth is a clue to the diagnosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree