Lung, Pleura, and Chest Wall
The conventional chest radiograph usually suffices for the detection and evaluation of most lung pathology. Despite its well-established role in detecting a wide spectrum of parenchymal disease, plain chest radiography has major limitations, particularly in the diagnosis of metastases, diffuse lung disease, and pleural and chest wall lesions. Due to its superior contrast sensitivity and cross-sectional imaging capability, computed tomography (CT) is ideally suited for the evaluation of the lung parenchyma, pleura, and osseous thorax (1,2). Ultrasound also may be valuable for the evaluation of the pleura and peripheral lung lesions. Magnetic resonance imaging (MRI) has virtually no role in the evaluation of parenchymal diseases, but it is a sensitive method for demonstrating chest wall abnormalities.
This chapter will discuss normal variants and the common abnormalities of the pediatric lung, pleura, diaphragm, and chest wall. These include neonatal pulmonary diseases, congenital lung anomalies, parenchymal tumors, pulmonary infection, diffuse pulmonary disease, pleural effusion, and chest wall abnormalities.
MEDICAL LUNG DISEASE OF THE NEONATE
A variety of pulmonary disorders occur in the newborn, some of which are medical and some of which are congenital anomalies (3,4). Hyaline membrane disease is the bestknown medical disorder. Wet lung, neonatal pneumonia, congenital lymphangiectasia, and chylothorax are the other important medical conditions.
HYALINE MEMBRANE DISEASE
Hyaline membrane disease (HMD), also known as respiratory distress syndrome, is a disorder of premature infants. It rarely occurs in infants beyond 37 weeks gestational age and then most commonly in infants of diabetic mothers. The disease is a consequence of pulmonary immaturity and consequent surfactant deficiency. Surfactant is a phospholipid compound that lines the alveolar cells and prevents atelectasis by lowering surface tension. Absence of surfactant leads to the development of atelectasis, inflammation, and epithelial necrosis, resulting in the hyaline membranes containing fibrin and cellular debris.
Hyaline membrane disease is a consequence of pulmonary immaturity and surfactant deficiency.
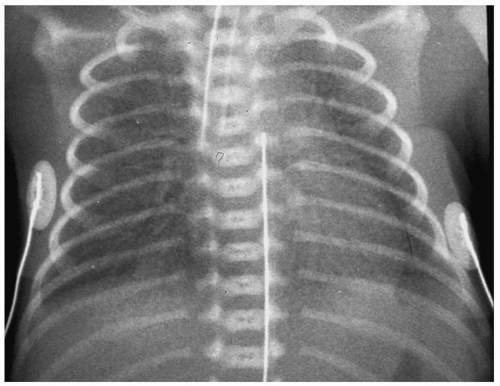
Infants with HMD are usually symptomatic shortly after birth, with signs of respiratory distress. Radiographic findings are usually seen within minutes after birth, and a normal
chest radiograph at 6 hours of age virtually excludes the diagnosis of HMD. Characteristic findings include hypoaeration and symmetric reticulogranular opacities, often with associated air bronchograms (Fig. 3.1). Typically, the radiographic changes worsen over the first 12 to 24 hours of life. In mild to moderate HMD, clearing of the granular infiltrates often begins at the end of the first week of life. Severe HMD is characterized by progressive opacification of the lungs. Treatment for HMD includes synthetic surfactant and ventilatory support.
chest radiograph at 6 hours of age virtually excludes the diagnosis of HMD. Characteristic findings include hypoaeration and symmetric reticulogranular opacities, often with associated air bronchograms (Fig. 3.1). Typically, the radiographic changes worsen over the first 12 to 24 hours of life. In mild to moderate HMD, clearing of the granular infiltrates often begins at the end of the first week of life. Severe HMD is characterized by progressive opacification of the lungs. Treatment for HMD includes synthetic surfactant and ventilatory support.
Radiographic findings of HMD include hypoaeration and symmetric reticulogranular opacities.
Barotrauma Complications of HMD
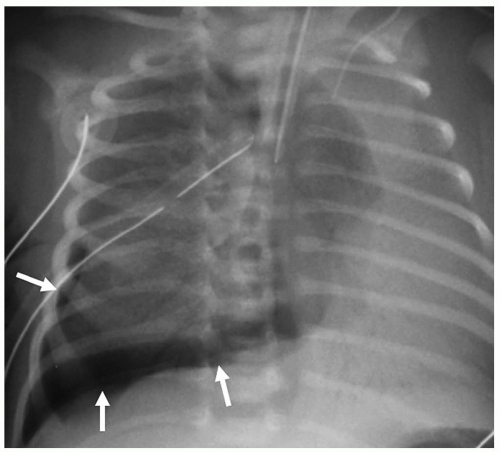
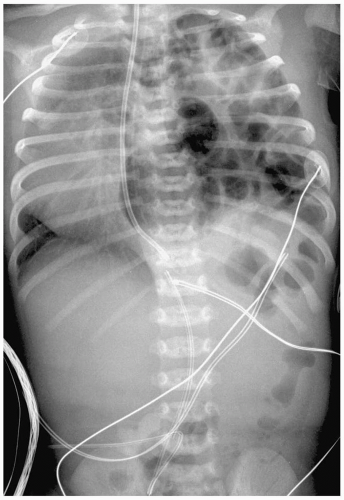
Complications of HMD and its treatment are extra-alveolar air collections and left-to-right shunting across a patent ductus arteriosus. Extra-alveolar air collections are the result of barotrauma and alveolar rupture and include pulmonary interstitial emphysema, pneumomediastinum, pneumopericardium, and pneumothorax. Pulmonary interstitial emphysema (PIE) results when there is alveolar rupture and subsequent dissection of air along the peribronchial and perivascular structures of the lung interstitium. PIE appears as irregular linear lucencies radiating from the hilar regions into the lung (Fig. 3.2). PIE results in fixed hyperinflation and may make ventilation difficult. In some cases, the air can coalesce and form a large pneumatocele or pulmonary pseudocyst.
Pulmonary interstitial emphysema (PIE) results when there is dissection of air along the structures of the lung interstitium.
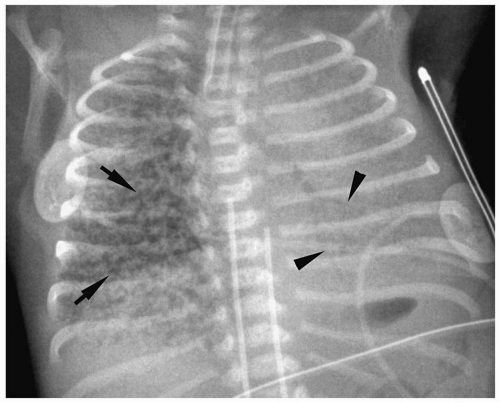
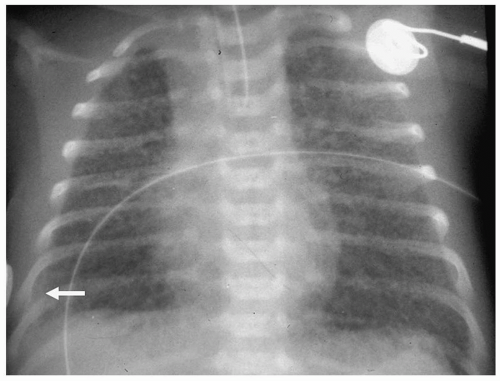
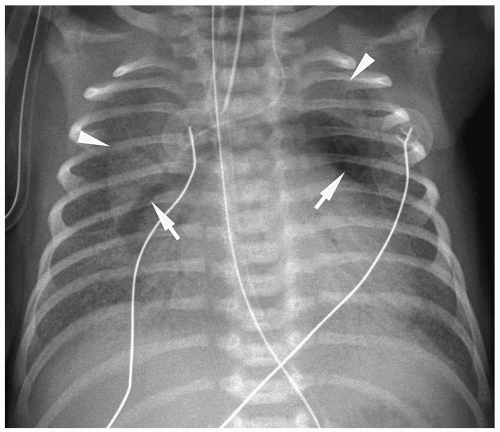 Figure 3.3 Pneumomediastinum. Radiograph in a preterm infant with respiratory distress syndrome shows lucencies (arrows) elevating the thymus (arrowheads), indicating pneumomediastinum. |
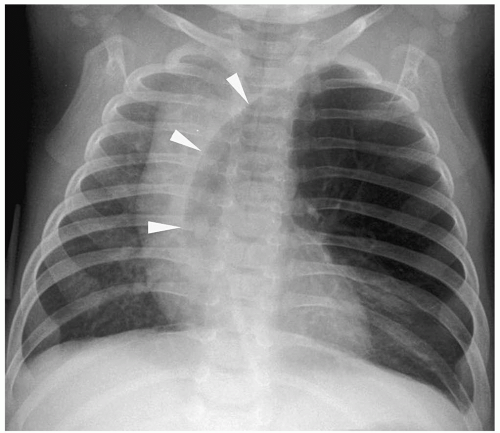
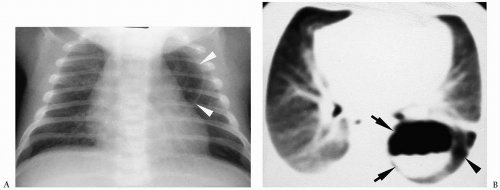
Pneumomediastinum appears as a radiolucent line around the heart border on the frontal radiograph and as a substernal air collection on the lateral radiograph. The air commonly extends beyond the origins of the great vessels at the pericardial reflection. The thymus may be elevated by the pneumomediastinum (Fig. 3.3). In pneumopericardium, air completely outlines the heart and does not extend past the pericardial reflection (Fig. 3.4). Pneumopericardium does not elevate the thymus.
The thymus may be elevated by the pneumomediastinum.
Pneumothorax may be the result of PIE or pneumomediastinum, which rupture into the pleural space. Pneumothorax is seen in up to 25% of infants on mechanical ventilation. Pneumothorax produces increased lucency of the involved hemithorax or a discrete pleural line (Fig. 3.5).
Pneumothorax is seen in up to 25% of infants on mechanical ventilation.
Persistent Patent Ductus Arteriosus
Normally, the ductus arteriosus closes 1 to 2 days after birth. Infants with HMD are usually hypoxemic and acidotic and have high levels of prostaglandin E1, causing the ductus to remain patent. In the first week of life, when pulmonary arterial pressure is physiologically increased, right-to-left shunting may occur across the patent ductus. With improvement in HMD, the pulmonary resistance drops, and shunting across the ductus becomes left-to-right. Left-to-right shunting may be recognized radiographically before clinical symptoms are apparent and is heralded by the development of an enlarging heart and/or pulmonary
edema (Fig. 3.6). Indomethacin has been used in some infants to promote ductal closure by inhibiting production of prostaglandins.
edema (Fig. 3.6). Indomethacin has been used in some infants to promote ductal closure by inhibiting production of prostaglandins.
Findings of a patent ductus arteriosus are an enlarging heart and pulmonary edema.
 Figure 3.4 Pneumopericardium. Frontal radiograph shows air within the pericardium (arrowheads) outlining the heart. The air extends only to the pericardial reflection. |
BRONCHOPULMONARY DYSPLASIA
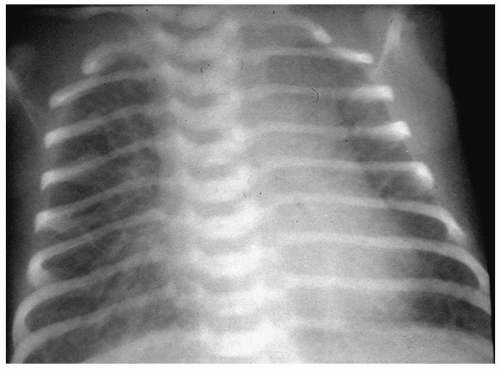
Bronchopulmonary dysplasia (BPD) is a delayed complication of assisted ventilation. It is the result of both oxygen toxicity and barotrauma. Radiographic findings include coarse infiltrates, cyst-like lucencies, and hyperaeration (Fig. 3.7). The radiographic findings usually improve and in most cases resolve by 3 to 5 years of age, although pulmonary function studies frequently remain abnormal.
BPD is the result of both oxygen toxicity and barotrauma.
Radiographic findings include coarse infiltrates, cyst-like lucencies, and hyperaeration.
WET LUNG DISEASE
The syndrome of wet lung disease (also known as transient tachypnea, transient respiratory distress of the newborn, and retained fluid syndrome) is a self-limited disorder that
primarily affects full-term infants. Delayed clearance of fetal lung fluid is the postulated mechanism for transient tachypnea. Factors predisposing to retained fluid include cesarean section (because of the absence of compression of the fetal thorax by the vaginal canal) and prolonged maternal administration of fluids or sedative drugs. Most patients are asymptomatic in the immediate postnatal period, but they develop respiratory distress within the first 6 hours of life. Symptoms usually peak at 24 to 36 hours and then decrease, eventually resolving by 2 to 3 days.
primarily affects full-term infants. Delayed clearance of fetal lung fluid is the postulated mechanism for transient tachypnea. Factors predisposing to retained fluid include cesarean section (because of the absence of compression of the fetal thorax by the vaginal canal) and prolonged maternal administration of fluids or sedative drugs. Most patients are asymptomatic in the immediate postnatal period, but they develop respiratory distress within the first 6 hours of life. Symptoms usually peak at 24 to 36 hours and then decrease, eventually resolving by 2 to 3 days.
Wet lung disease primarily affects full-term infants.
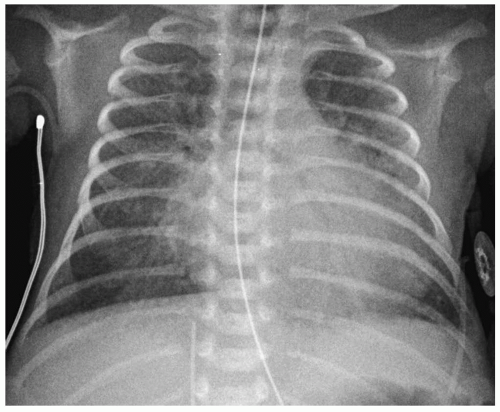 Figure 3.6 Patent ductus arteriosus. Chest radiograph of a newborn with a murmur shows cardiomegaly with left ventricular prominence and pulmonary overcirculation. |
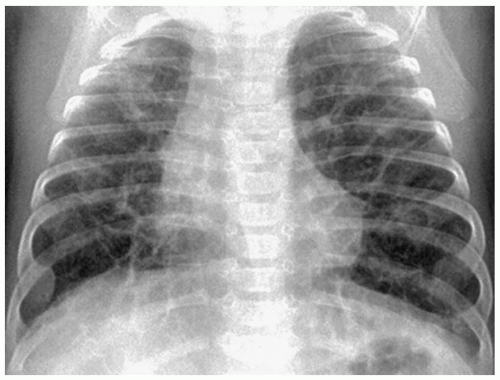 Figure 3.7 Bronchopulmonary dysplasia. Frontal radiograph shows coarse interstitial opacities with focal areas of emphysema. |
Chest radiographic findings of wet lung disease are increased vascular markings, linear interstitial opacities, and pleural effusions, reflecting interstitial edema (Fig. 3.8). On occasion, patchy areas of alveolar opacification, corresponding to airspace edema or atelectasis, may be seen. The lungs are normally inflated to slightly hyperaerated. Cardiac size usually is normal. Radiographic findings resolve within 48 to 72 hours.
Radiographic findings of wet lung disease are increased vascular markings, interstitial opacities, and pleural effusions.
The differential diagnostic considerations for reticular opacities in the newborn include total anomalous pulmonary venous connection with obstruction, primary pulmonary lymphangiectasia, and streptococcal pneumonia. Normal blood gases, prompt improvement with oxygen therapy, and progressive clearing of the lung disease on serial radiographs militate against heart or lymphatic disease as the cause of the radiographic abnormalities. Negative cultures exclude neonatal pneumonia.
Differentiation between wet lung syndrome and mild HMD may be difficult if radiographs are obtained soon after birth. Infants under 2 hours of age with HMD frequently
have interstitial or alveolar fluid. Serial radiographs can help in differentiating between the two conditions. By 4 to 6 hours, infants with HMD usually demonstrate hypoaeration as well as granular lung disease, whereas those infants with wet lung syndrome usually show hyperinflation.
have interstitial or alveolar fluid. Serial radiographs can help in differentiating between the two conditions. By 4 to 6 hours, infants with HMD usually demonstrate hypoaeration as well as granular lung disease, whereas those infants with wet lung syndrome usually show hyperinflation.
MECONIUM ASPIRATION
Meconium aspiration syndrome is the result of aspiration of meconium at the time of delivery. It most commonly affects neonates who are postmature. Intrauterine fetal distress causes passage of meconium and fetal gasping, which leads to meconium aspiration. The diagnosis is usually known clinically, as meconium will have been suctioned from below the vocal cords at delivery. Aspiration of meconium causes partial or complete obstruction of both medium and small airways, with resultant subsegmental atelectasis associated with overinflation of the lungs. Because meconium is an irritant to the bronchial mucosa, chemical pneumonitis also occurs.
Meconium aspiration syndrome is the result of intrauterine fetal distress.
The radiographic findings of meconium aspiration are hyperinflation and bilateral, interstitial and airspace opacities (Fig. 3.9). Alveolar rupture and air-block complications, such as pneumothorax or pneumomediastinum, occur in 25% to 40% of affected patients. Treatment is supportive, consisting of oxygen therapy and extracorporeal membrane oxygenation (ECMO).
Radiographic findings are interstitial and airspace opacities and hyperinflation.
NEONATAL PNEUMONIA
Pneumonia occurs in less than 1% of live-born infants. Neonatal pneumonia may be acquired in utero during labor, at delivery, or shortly after birth. The major risk factor for intrauterine development of pneumonia is prolonged rupture of the membranes.
Group B Streptococcus is the most common cause of neonatal pneumonia. Clinical features are similar to those of HMD, that is, mild to moderate respiratory distress. Radiographic findings include reticulogranular opacities similar to those in HMD and pleural effusions. The latter occur in about 65% of patients (Fig. 3.10). Pleural effusions do not occur with HMD.
Radiographic findings in neonatal pneumonia are reticulogranular opacities and pleural effusions.
Chlamydial pneumonia caused by Chlamydia trachomatis is acquired from the mother by direct contact during vaginal delivery. Pneumonia typically develops between 2 weeks and 3 months of age, and the average age of onset is 6 weeks. Approximately 50% of infants with chlamydial pneumonia have conjunctivitis. Radiographic abnormalities include hyperinflation and patchy, asymmetric interstitial and alveolar opacities (Fig. 3.11). Pleural effusions are uncommon.
Radiographic abnormalities in chlamydial pneumonia include interstitial and alveolar opacities.
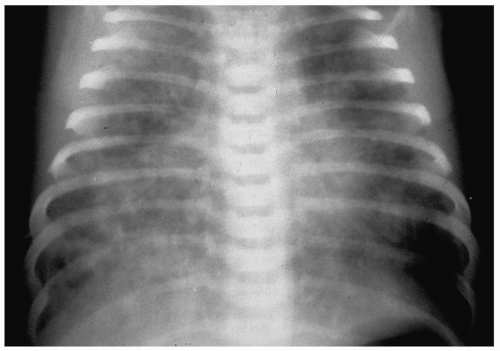 Figure 3.9 Meconium aspiration. Frontal radiograph in a newborn with fetal distress shows diffuse patchy airspace disease. |
Less common causes of neonatal pneumonia are Pseudomonas, Proteus, Klebsiella, and Enterobacter organisms. Radiographic findings are nonspecific and include bilateral, patchy, pulmonary opacities with associated hyperinflation.
CONGENITAL PULMONARY LYMPHANGIECTASIA
Lymphangiectasia of the lung refers to abnormal dilatation of the pulmonary lymphatics (5). This condition may be a primary anomaly, due to an error in pulmonary lymphatic development with persistence of dilated lymphatic channels, or it may be associated with generalized lymphangiomatosis or severe pulmonary venous obstruction (e.g., hypoplastic left heart syndrome, total anomalous pulmonary venous return, cor triatriatum, or pulmonary vein atresia).
Lymphangiectasia of the lung may be a primary anomaly or associated with generalized lymphangiomatosis or pulmonary venous obstruction.
Patients with primary lymphatic disease present with severe respiratory distress and cyanosis in the neonatal period. Most do not survive. Patients with venous obstruction present with symptoms related to the underlying cardiac disease. Patients with generalized lymphangiectasia usually are asymptomatic.
Characteristic radiographic findings of pulmonary lymphangiectasia are coarsely nodular or reticular opacities, Kerley B lines, and pulmonary overaeration. Pleural effusions are common (Fig. 3.12). Although the disease process usually involves all of both lungs, rarely it may be localized to one lobe.
 Figure 3.11 Chlamydial infection. Frontal radiograph in a 4-week-old child shows diffuse interstitial opacities. |
CHYLOTHORAX
Chylothorax is the abnormal accumulation of lymph in the pleural space, and it is the most common cause of a large pleural effusion in the neonate. It usually results from thoracic duct rupture during delivery, which allows leakage of chyle into the pleural spaces. Less often, it is due to a congenital abnormality of the mediastinal and pulmonary lymphatics. Neonates with chylothorax are usually term infants. Approximately 50% of patients present with signs of respiratory distress on the first day of life, and the remainder present by the end of the first week.
Chylothorax is the most common cause of a large pleural effusion in the neonate.
Radiographic findings of chylothorax include a pleural effusion, usually unilateral (Fig. 3.13). Chylothorax is most common on the right, but left-sided or bilateral chylothorax can occur. In patients with large effusions, there is complete opacification of the hemithorax with contralateral shift of the heart and mediastinum. Chylous ascites may be present. Rarely, there is associated pulmonary hypoplasia due to compression of the developing lung in utero by a large chylothorax.
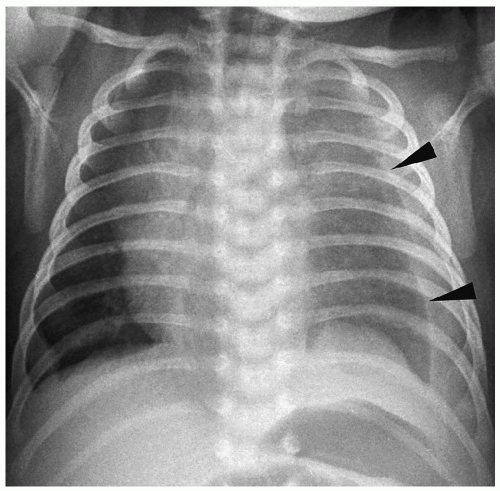 Figure 3.13 Chylous effusion. Frontal radiograph in a newborn with respiratory distress shows a large left pleural effusion (arrowheads). |
Thoracentesis usually yields cloudy fluid because of the high lipid content of chyle, although the aspirated fluid will be clear prior to the initiation of feedings. Treatment includes thoracentesis, chest tube drainage, and feedings of medium-chain triglycerides. Most lymphatic ruptures seal with combined chest tube and dietary treatment.
The differential diagnosis of chylothorax includes the nonchylous pleural effusions, such as wet lung disease, hydrops fetalis, Turner syndrome, pulmonary vein obstruction, congestive heart failure, and esophageal rupture.
CONGENITAL LUNG ANOMALIES
Congenital pulmonary masses can be classified into two major categories: those with normal arterial supply and venous drainage and those with anomalous vasculature. CT and sometimes MRI can be useful to clarify the diagnosis or to determine the extent of abnormality in patients in whom surgery is contemplated.
ANOMALIES WITH NORMAL VASCULATURE
Congenital Lobar Emphysema
Congenital lobar emphysema is a condition characterized by hyperinflation of a lobe without destruction of alveolar septa (6). Bronchial obstruction is thought to be the cause of emphysema. Reasons for obstruction include primary cartilage deficiency or dysplasia; intraluminal mucosal folds, webs, or stenoses; and extrinsic compression by a vessel or mass. The left upper lobe is involved in about 45% of cases, the right middle lobe in 30%, the right upper lobe in 20%, and two lobes in 5% of cases. Most patients present during the first 6 months of life with dyspnea or cyanosis. Occasionally, the diagnosis is unsuspected until later in life when patients present with wheezing or cough. About 30% of patients have associated congenital heart lesions, usually ventricular septal defect or patent ductus arteriosus.
Congenital lobar emphysema is characterized by hyperinflation of a lobe without destruction of alveolar septa.
Characteristic radiographic and CT findings include a hyperinflated lucent lobe without a perceptible wall, flattening of the ipsilateral hemidiaphragm, atelectasis of adjacent lobes, and mediastinal shift (Figs. 3.14 and 3.15). In the first 1 or 2 days of life, the overdistended lobe may appear opaque rather than lucent, because it is fluid-filled as a result of impaired clearance of amniotic fluid. As the fluid is resorbed through vascular and lymphatic channels, the classic radiographic appearance develops. Lobectomy is often needed
in patients with severe dyspnea. Patients with milder symptoms may be observed; in some instances the hyperinflated lobe involutes spontaneously.
in patients with severe dyspnea. Patients with milder symptoms may be observed; in some instances the hyperinflated lobe involutes spontaneously.
In the first 1 or 2 days of life, the overdistended lobe may appear opaque rather than lucent.
The differential diagnosis of a lucent hemithorax or hyperinflated lung in the neonate includes cystic adenomatoid, bronchogenic cyst, diaphragmatic hernia, pneumothorax (all discussed below), and also pulmonary sling. In pulmonary sling, the anomalous vessel courses either over the right mainstem bronchus, resulting in hyperinflation of the entire right lung, or over the intermediate bronchus, resulting in hyperinflation of the right middle and lower lobes (7) (Fig. 3.16).
Cystic Adenomatoid Malformation
Cystic adenomatoid malformation consists of a mass of cysts lined by bronchial or cuboidal epithelium. Histologically, three types of malformations have been described: type I (50% of cases) contains a single or multiple large cysts (>2 m in diameter); type II (41%) contains multiple small cysts (1 to 10 mm in diameter); type III (9%) is a solid lesion to visual inspection but contains microscopic cysts (8,9,10). All varieties communicate with the
tracheobronchial system and have normal vascular supply and drainage. The anomaly occurs with equal frequency in both lungs, with slight upper lobe predominance. Rarely, multiple lobes are involved. Most patients present in the first 6 months of life with respiratory distress. After the neonatal period, affected children present with cough, fever, or recurrent pulmonary infections. Associated anomalies of the kidneys, bowel, heart, and skeletal system are common in types II and III malformations.
tracheobronchial system and have normal vascular supply and drainage. The anomaly occurs with equal frequency in both lungs, with slight upper lobe predominance. Rarely, multiple lobes are involved. Most patients present in the first 6 months of life with respiratory distress. After the neonatal period, affected children present with cough, fever, or recurrent pulmonary infections. Associated anomalies of the kidneys, bowel, heart, and skeletal system are common in types II and III malformations.
Three types of malformations have been described.
 Figure 3.16 Pulmonary sling. Chest radiograph shows hyperinflation of the right middle and lower lobes with diminished vascular markings and mediastinal shift. |
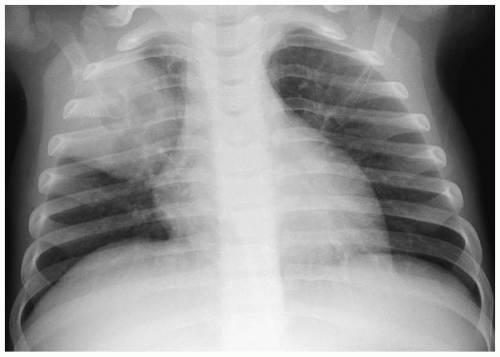 Figure 3.17 Cystic adenomatoid malformation. Chest radiograph shows right upper lobe opacity with central lucency. |
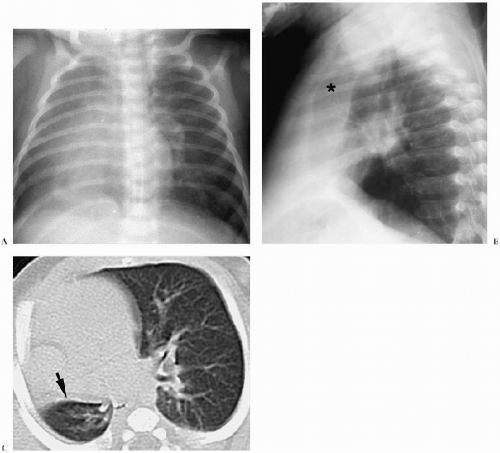
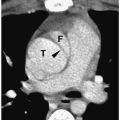
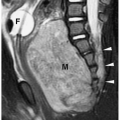
In the neonate, the typical chest radiographic finding is a complex mass containing (Fig. 3.17) an admixture of cystic and solid components. On CT, cystic adenomatoid malformation appears as a large mass containing multiple, variable-sized, thin-walled cysts (Fig. 3.18). When infected, the cyst contents increase in attenuation and the walls become thicker. Air-fluid levels may occasionally be noted. Treatment is surgical resection. Spontaneous in utero resolution of cystic adenomatoid malformation has been reported.
The typical chest radiographic finding of cystic adenomatoid malformation is a complex mass.
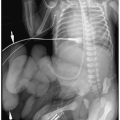
A congenital diaphragmatic or Bochdalek hernia needs to be considered in the differential diagnosis of a multicystic intrathoracic mass. Bochdalek hernias are usually left-sided (90% of cases). Rarely, they are right-sided or bilateral. Radiographic findings of diaphragmatic hernia are intrathoracic air-filled loops of bowel, contralateral mediastinal shift, and a relative paucity of intra-abdominal bowel gas (Fig. 3.19). If radiographs are obtained before swallowed air has entered the bowel, the herniated intestines may appear opaque rather than lucent. In these cases, placement of a nasogastric tube can demonstrate an abnormal location of the bowel in the chest. Delayed films also can confirm the presence of air within the intrathoracic bowel.
Bochdalek hernia needs to be considered in the differential diagnosis of a multicystic intrathoracic mass.
Intrapulmonary Bronchogenic Cyst
Intrapulmonary bronchogenic cysts are surrounded by fibrous walls containing cartilage and lined by ciliated, columnar epithelium. They become clinically apparent when there is superimposed infection or compression of the tracheobronchial tree.
Pulmonary bronchogenic cysts commonly appear opaque on plain radiographs and have attenuation values equal to that of water on CT, reflecting the presence of fluid contents (Fig. 3.20). The attenuation values can increase if the contents are proteinaceous or mucoid. Air or air-fluid levels can be seen within the cysts if they communicate with the airways. On MRI, the cyst appears hypointense on T1-weighted spin-echo images and hyperintense on T2-weighted and fat-suppressed sequences.
Bronchogenic cysts appear opaque on plain radiographs and have CT attenuation values equal to that of water.
Pulmonary Agenesis and Hypoplasia
Pulmonary agenesis refers to absence of lung tissue. Patients with bilateral involvement die almost immediately. When the involvement is unilateral, patients can present with respiratory distress immediately after delivery or they may be asymptomatic and the
diagnosis made incidentally on imaging examinations obtained for other reasons. Radiographic studies show an opacified hemithorax, shift of mediastinal structures to the affected side, and compensatory hyperinflation of the normal side.
diagnosis made incidentally on imaging examinations obtained for other reasons. Radiographic studies show an opacified hemithorax, shift of mediastinal structures to the affected side, and compensatory hyperinflation of the normal side.
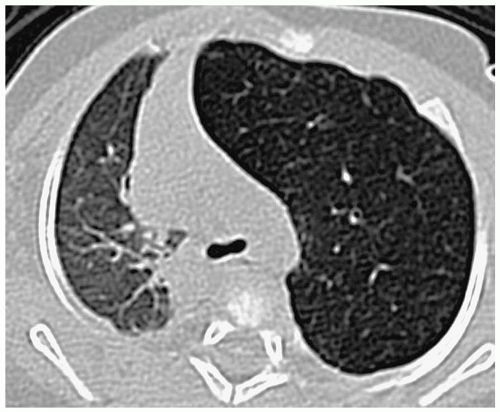
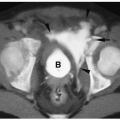
Pulmonary hypoplasia refers to a decreased amount of lung tissue. Pulmonary hypoplasia can be primary with no obvious cause of underdevelopment or it can occur secondary to extrathoracic compression of the fetal lung (usually due to maternal oligohydramnios), thoracic cage compression of the fetal lung (usually due to thoracic dystrophies), and intrathoracic fetal compression of lung (usually due to large diaphragmatic hernias or large congenital cystic masses). Patients may have mild respiratory distress or no symptoms at all. In comparison with the agenetic lung, imaging studies show some aeration. The hypoplastic lung is smaller than normal, and there is associated pulmonary artery hypoplasia and some mediastinal shift. Increased soft tissue density may be noted anterior to the hypoplastic lung on the lateral radiograph (Fig. 3.21).
CT findings of pulmonary hypoplasia are a small lung and pulmonary artery and mediastinal shift.
Bronchial Atresia
Bronchial atresia (also known as congenital bronchocele) results from abnormal development of a segmental or subsegmental bronchus. The bronchus distal to the atretic segment
is patent and contains impacted mucus, and the lung beyond the obstruction is air-filled and overaerated, as a result of collateral air drift via the pores of Kohn. Bronchial atresia rarely causes symptoms and is usually discovered on chest radiographs performed for other indications. The radiographic finding of bronchial atresia is a nodule. The CT features of bronchial atresia include a dilated, mucoid-filled segmental or subsegmental bronchus surrounded by hyperinflated (oligemic) lung (Fig. 3.22).
is patent and contains impacted mucus, and the lung beyond the obstruction is air-filled and overaerated, as a result of collateral air drift via the pores of Kohn. Bronchial atresia rarely causes symptoms and is usually discovered on chest radiographs performed for other indications. The radiographic finding of bronchial atresia is a nodule. The CT features of bronchial atresia include a dilated, mucoid-filled segmental or subsegmental bronchus surrounded by hyperinflated (oligemic) lung (Fig. 3.22).
Features of bronchial atresia include a dilated, mucoid-filled bronchus surrounded by hyperinflated lung.
ANOMALIES WITH ABNORMAL VASCULATURE
Bronchopulmonary Sequestration
Pulmonary sequestration refers to a portion of lung that has no normal connection with the tracheobronchial tree and is supplied by an anomalous artery, usually arising from the aorta (11,12,13). When the sequestered lung is confined within the normal visceral pleura and has venous drainage to the pulmonary veins, it is termed intralobar, or acquired. The
sequestered lung is termed extralobar, or congenital, when it has its own pleura and venous drainage to systemic veins. Intralobar sequestrations constitute approximately 75% of all sequestrations.
sequestered lung is termed extralobar, or congenital, when it has its own pleura and venous drainage to systemic veins. Intralobar sequestrations constitute approximately 75% of all sequestrations.
Intralobar sequestrations account for approximately 75% of all sequestrations.
Patients with intralobar sequestration typically present with chronic or recurrent segmental or subsegmental pneumonitis, especially at a lung base. Extralobar sequestration is often an incidental finding on radiographs obtained for other clinical indications. Extralobar sequestration can be associated with pleural effusions, hydrops and polyhydramnios, cystic adenomatoid malformation, usually type II, and Bochdalek hernia.
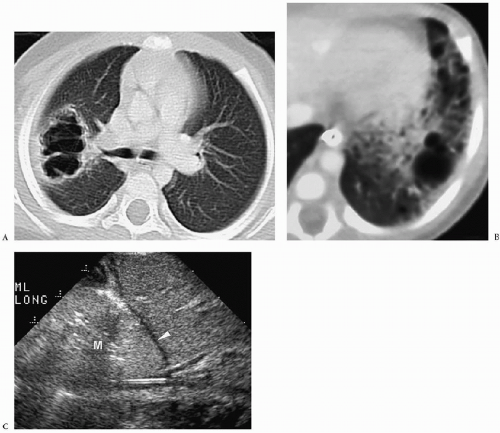
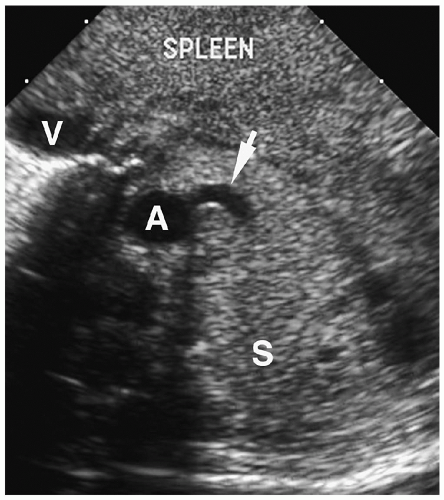
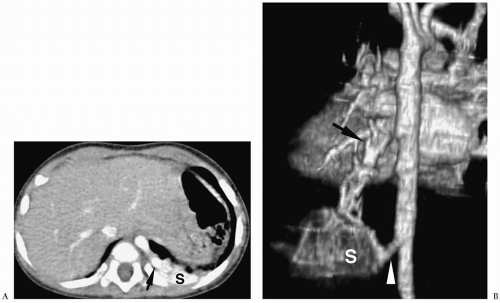
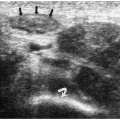
Chest radiographic findings include a focal opacity or infiltrate with or without air bronchograms (Fig. 3.23). Occasionally, radiographs may demonstrate an anomalous vessel, but sonography, CT, and MRI are more sensitive for identifying such a vessel. Ultrasonography is useful in neonates to show the feeding artery (Fig. 3.24). In older patients, the
sequestered lung, feeding arteries, and draining veins are easier to identify with CT or MRI (Figs. 3.25 and 3.26) (14). CT scanning after an injection of intravenous contrast material demonstrates opacification of the anomalous artery immediately after aortic enhancement. The CT appearance of the pulmonary parenchyma depends on whether or not the sequestered lung is aerated. When the sequestration communicates with the remainder of the lung, usually after being infected, it appears cystic; a sequestration that does not communicate appears as a homogeneous density, usually in the posterior portion of the lower lobe. Hyperinflated or emphysematous lung often surrounds the sequestered lung. On MRI, the feeding vessel appears as an area of signal void on T1-weighted spin-echo images and as a hyperintense area on gradient echo sequences. The parenchymal portion of the sequestration appears as an area of intermediate or high signal intensity.
sequestered lung, feeding arteries, and draining veins are easier to identify with CT or MRI (Figs. 3.25 and 3.26) (14). CT scanning after an injection of intravenous contrast material demonstrates opacification of the anomalous artery immediately after aortic enhancement. The CT appearance of the pulmonary parenchyma depends on whether or not the sequestered lung is aerated. When the sequestration communicates with the remainder of the lung, usually after being infected, it appears cystic; a sequestration that does not communicate appears as a homogeneous density, usually in the posterior portion of the lower lobe. Hyperinflated or emphysematous lung often surrounds the sequestered lung. On MRI, the feeding vessel appears as an area of signal void on T1-weighted spin-echo images and as a hyperintense area on gradient echo sequences. The parenchymal portion of the sequestration appears as an area of intermediate or high signal intensity.
Radiographic findings of sequestration include focal opacity or infiltrate with or without air bronchograms.
 Figure 3.23 Pulmonary sequestration in a boy with recurrent pneumonia. Frontal radiograph demonstrates right lower lobe opacity with air fluid levels (arrowhead). |
In pulmonary sequestration, CT demonstrates opacification of the anomalous artery immediately after aortic enhancement.
Hypogenetic Lung Syndrome
Hypogenetic lung syndrome, also known as venolobar syndrome or scimitar syndrome, refers to anomalous pulmonary venous return accompanying hypoplasia of the right lung and its pulmonary artery (15,16). The anomalous venous return is most often to the inferior vena cava, but it may be supra- or intracardiac. Associated cardiovascular anomalies occur in approximately 25% of patients, the most common being atrial septal defect, followed by ventricular septal defect, tetralogy of Fallot, and patent ductus arteriosus. Bronchial anomalies are also common, particularly isomerism. The presence or absence of symptoms relates to the severity of pulmonary hypoplasia and the complexity of the cardiac malformations.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree