Chapter 29 Hematologic Malignancy
The Lymphomas
Epidemiology
Incidence
In the United States, NHL annually accounts for 5% of new cancers in men and 4% of new cancers in women. It is estimated that there were 66,360 new cases of NHL and 19,320 deaths in 2011.1 HL accounts for approximately 15% of all lymphomas. It is estimated that there will be 8830 new cases in 2011 and 1300 deaths.1 Men are affected somewhat more than women: for HL, the male-to-female ratio is 1.4:1; for NHL, it is 1.1:1.
The incidence of HL has been stable for years, but that of NHL has risen by approximately 60% in the United States since 1960. This is evident for all age groups, but particularly for older persons. It occurs more often in men and in white ethnic groups, but has been observed in all geographic areas of the United States.2 Geographically, the incidence of NHL varies 8- to 10-fold, being much more common in the West. The overall mortality for NHL has also risen over the last few decades, especially in older patients, despite the fact that survival rates for each subtype of NHL have improved, reflecting advances in treatment.
The reasons for the increasing incidence are only partly understood. Some may be artifactual, in that new lymphoma classifications have led to a diagnosis of NHL in patients who would previously have had other diagnoses,3 including HL in up to 15% of cases. Part of the increase from the late 1980s resulted from the increased incidence of lymphomas associated with immune deficiency, notably secondary to human immunodeficiency virus (HIV) infection. However, the occurrence of some HIV-associated NHLs has started to fall since the introduction of highly active antiretroviral therapies.4
Key Points Incidence
• Incidence of NHL has increased by 60% since the 1960s in the United States and the United Kingdom.
• HL has a peak incidence between the ages of 20 and 40 years and also in those older than 50 years.
• NHLs are seen in children and in those older than 50 years.
• There is a link between Epstein-Barr virus (EBV) and lymphoma, especially Burkitt lymphoma (BL) and HL.
Etiology
There is an association between EBV and HL, but the exact etiologic role of the virus is uncertain. Patients with HL have a higher antibody titer to EBV viral capsular antigen, and the risk of HL among patients who have had infectious mononucleosis is trebled. EBV can also be found in the malignant cells of HL. Other infective agents such as human herpesvirus-6 (HHV-6) and HIV-1, both associated with the mixed cellularity subtype of classic HL, may also have roles in development of this disease. Infective agents are also associated with the development of certain subtypes of NHL. EBV is found in virtually 100% of cases of endemic African BL; in the sporadic form, the incidence is 15% to 30%. The rare primary effusion lymphomas are associated with HHV-8. Helicobacter pylori infection is necessary for the development of gastric lymphoma of mucosa-associated lymphoid tissue (MALT) type. The human T-cell lymphotrophic virus (HTLV-1) retrovirus has a causal relationship with adult T-cell leukemia/lymphoma, seen in South Japan and the Caribbean. The disease is believed to represent clonal expansion of HTLV-1–infected T lymphocytes.
Pathology
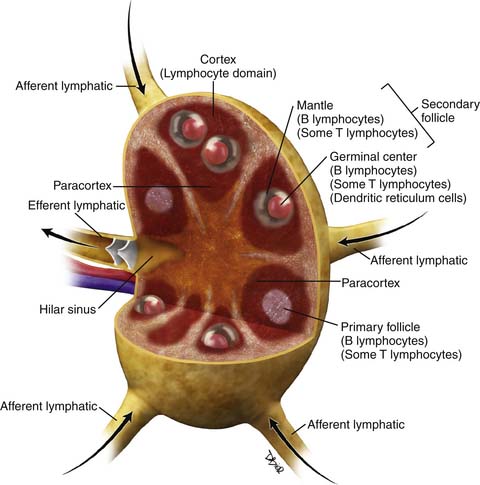
There have been several pathologic classifications of lymphoma in the past; however, the functional anatomy of the lymph node is key to understanding the pathologic classification of lymphomas (Figure 29-1).
Non-Hodgkin Lymphoma
Over 90% of NHLs in the Western world are of B-cell origin. In general, those arising at stages of development within the germinal center of the node have a follicular or nodular architecture, whereas those arising outside the germinal center have a diffuse pattern. New insights into the pathogenesis of NHL, in terms of cellular and immunologic origins, have resulted in the introduction of the Revised European American Classification of Lymphoid Neoplasms (REAL) classification in 1994. This, in turn, led to the adoption of the definitive World Health Organization (WHO) Classification of Tumors of Hematopoietic and Lymphoid Tissues in 1995,5 which is an updated version of the REAL classification (Table 29-1).
Table 29-1 Summary of the World Health Organization Classification of Tumors of Lymphoid Tissue
ALK, anaplastic lymphoma kinase; BL, Burkitt lymphoma; CNS, central nervous system; CLL, chronic lymphocytic leukemia; DLBCL, diffuse large B-cell lymphoma; EBV, Ebstein-Barr virus; HHV-8, human herpesvirus-8; HL, Hodgkin lymphoma; LBCL, large B-cell lymphoma; MALT, mucosa-associated lymphoid tissue; NK, natural killer; NOS, not otherwise specified; PTLD, posttransplant lymphoproliferative disorder.
The WHO classification is a list of distinct disease entities defined by a combination of morphology, immunophenotype, and genetic and clinical features. It stratifies neoplasms according to myeloid, lymphoid, and histiocytic/dendritic cell lineages and recognizes three major categories of lymphoid neoplasms: B cell, T cell, and natural killer (NK) cell; and separately, HL. Precursor neoplasms, corresponding to the early stages of differentiation and including lymphoblastic leukemias and lymphomas, are separated from the more mature or peripheral neoplasms. Conversely, chronic lymphocytic leukemia (CLL) is the circulating form of small lymphocytic lymphoma, a mature B-cell neoplasm, and is classified as such. Histologic grade may influence therapeutic decision-making. For example, follicular lymphoma (FL) is graded according to the number of centroblasts per high-power field.
This comprehensive approach has significantly improved the consistency of classification of lymphoma, such that, if given sufficient material, expert hematopathologists agree on classification of entities in over 95% of cases.6 Further refinements may facilitate appropriate patient management.7 For example, gene expression profiling for diffuse large B-cell lymphoma (DLBCL) enables recognition of discrete subsets (germinal center B-cell type and activated B-cell type) that have independent prognostic significance.8,9 Other recent additions to the classification include pediatric FL, primary DLBCL of the central nervous system (PCNSL), and two so-called gray zone lymphomas: B-cell lymphoma with features intermediate between DLBCL and classic HL, and B-cell lymphoma with features intermediate between DLBCL and BL.
Hodgkin Lymphoma
Investigators have demonstrated that HL is a true lymphoma; hence, the term Hodgkin lymphoma is preferred to Hodgkin disease. Indeed, the distinction between HL and NHL is not always straightforward, and composite cases occur. Diagnosis depends upon the demonstration of malignant Reed-Sternberg and Hodgkin cells against a background of non-neoplastic inflammatory cells. The WHO classification recognizes two distinct entities that differ in clinical features, behavior, morphology, and immunophenotype: classical Hodgkin lymphoma (CHL, 95%) and nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL, 5%). CHL is, in turn, divided into four subgroups, based on the proportion of lymphocytes in relation to the number of malignant cells and on the connective tissue background (Table 29-2).10 All share the same immunophenotype, with expression of CD30 by the malignant cells.
Table 29-2 Rye Classification of Classical Hodgkin Lymphoma and Approximate Frequency
| HISTOLOGY | FREQUENCY (%) |
|---|---|
| Lymphocyte rich | 5 |
| Nodular sclerosis | 65 |
| Mixed cellularity | 25 |
| Lymphocyte depletion | 5 |
From Harris NL, Jaffe ES, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting—Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17:3835-3849.
Key Points Classification of lymphomas
• HL comprises two distinct entities: NLPHL (5%) and CHL (95%).
• CHL consists of four subtypes: nodular sclerosing (70%), mixed cellularity (20-25%), lymphocyte-rich (5%), and lymphocyte-depleted (<5%).
• WHO classification stratifies lymphoma according to myeloid, lymphoid, and histiocytic/dendritic lineage and provides distinction between disease entities.
Clinical Features
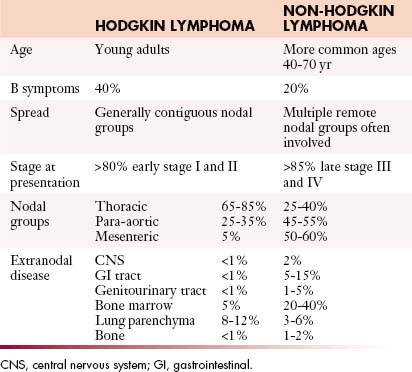
Both HL and NHL are predominantly diseases of lymph nodes, which may present as a localized process involving a single nodal group or organ or as widely disseminated disease. However, there are recognizable differences in the clinical presentation of the two diseases (Table 29-3).
Table 29-3 Key Differences between the Clinical Features of Hodgkin Lymphoma and Non-Hodgkin Lymphoma
Hodgkin Lymphoma
Most patients with HL present with painless asymmetrical lymph node enlargement, which may be accompanied by sweats, fever, weight loss (“B” symptoms), and pruritus in approximately 40% of patients. B symptoms are more common in advanced-stage disease and thus in the mixed cellularity and lymphocyte-depleted subtypes of CHL. Alcohol-induced pain is rare.
Staging Systems and Prognostication
Because lymphomas are primarily neoplasms of lymphoid tissues (whether nodal or extranodal), the tumor-node-metastasis (TNM) staging system is not appropriate. The Ann Arbor staging system for HL was introduced in 1970 and took into account the extent of nodal disease and the presence of extranodal extension. Increasing recognition of the influence of tumor bulk as an independent prognostic indicator within each stage and the routine application of computed tomography (CT) in the 1980s led to a modification of the classification in 1989, the Cotswolds classification.11 This system is similar to the original Ann Arbor classification, but stage III is subdivided and an additional qualifier “X” denotes bulky disease (Table 29-4). The prognosis of HL depends upon a number of factors, including
• Age. Older patients have a worse prognosis (for early-stage disease, 5-year survival is 45% in patients older than age 65, as opposed to over 90% in younger patients).
• Tumor subtype. Mixed cellularity and lymphocyte-depleted HL have a poorer prognosis.
• Raised erythrocyte sedimentation rate (ESR).
• Multiple sites of involvement (more than three or four involved regions).
Table 29-4 Staging of Lymphoma (Cotswolds Classification)
| STAGE | AREA OF INVOLVEMENT | |
|---|---|---|
| I | One lymph node region or extralymphatic site | |
| II | Two or more lymph node regions on the same side of the diaphragm | |
| III | ||
| IV | Extranodal sites beyond those designated “E” | |
| Additional qualifiers | A | No symptoms |
| B | Fever, sweats, weight loss (to 10% body weight) | |
| E | Involvement of a single extranodal site, contiguous in proximity to a known nodal site | |
| X | Bulky disease. Mass >10 cm maximum dimension | |
| CS | Clinical stage | |
| CE1 | Pathologic stage. Denoted by a subscript at a given site (M, marrow; H, liver; L, lung; O, bone; P, pleural; D, skin; S, spleen) | |
From Lister TA, Crowther D, Sutcliffe SB, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol. 1989;7:1630-1636.
The modified Ann Arbor classification is also currently applied to NHL, but is less useful, because the prognosis in NHL is more dependent on the histologic subtype, tumor bulk, and specific organ involvement, as well as stage. In NHL, the critical questions are whether or not disease is limited, the effect of the disease on end organs such as bone marrow, and symptomatology. Because NHL spreads more randomly than HL, the Ann Arbor staging system is less helpful in defining prognostic subgroups. Therefore, considerable effort has been applied to the development of robust prognostic indices that are accurate, simple, and discriminant. Such indices not only aid in the management of the individual patient but also facilitate meaningful comparison of results from clinical trials. The International Prognostic Index (IPI) was developed by an international collaborative group.12 For DLBCL, five factors have prognostic significance: age older than 60 years, elevated serum lactate dehydrogenase (LDH), Eastern Cooperative Oncology Group (ECOG) performance status greater than 1 (i.e., nonambulatory), advanced stage (III or IV) disease, and more than one extranodal site of disease.
Four risk groups are recognized depending on the number of adverse prognostic features that are present. Such a stratification enables choice of more intensive therapies for those at higher risk. Prior to the monoclonal antibody era, patients in the low risk group (no or one prognostic factor present) had a 5-year survival greater than 70%, whereas patients in the high risk group (four or five factors present) had only a 25% 5-year survival. More recently, the prognostic importance of gene expression profiling within individual subtypes of NHL has become evident, as described previously for DLBCL,8 where the better prognosis of the germinal center–like subtype compared with the activated B-cell subtype is independent of the IPI. A similar prognostic index has been developed for FL,13 where the important factors are age older than 60, elevated serum LDH, hemoglobin less than 12 g/dL, stage 3 or 4 disease, and more than four nodal sites of disease.
The clinical spectrum of childhood lymphoma differs somewhat from adult lymphoma with more frequent extranodal involvement, especially of the gastrointestinal tract, solid viscera including the kidneys and pancreas, and extranodal sites in the head and neck.14 In these situations, the St. Jude or Murphy’s staging classification is applied, because it takes into account the increased frequency of extranodal disease (Table 29-5).
Table 29-5 Murphy’s Staging System for Childhood Non-Hodgkin Lymphoma
| STAGE | CRITERIA FOR EXTENT OF DISEASE |
|---|---|
| I | A single tumor (extranodal) or single nodal region except in the mediastinum or abdomen |
| II | A single tumor (extranodal) with regional nodal involvement Two or more nodal areas on the same side of the diaphragm Two single (extranodal) tumors with or without regional node involvement on the same side of the diaphragm A primary GI tract tumor, usually ileocecal, with or without involvement of associated mesenteric nodes only, grossly completely resected |
| III | Two single tumors (extranodal) on opposite sides of the diaphragm Two or more nodal areas above and below the diaphragm ALL primary intrathoracic tumors (mediastinal, pleural, thymic) ALL extensive primary intra-abdominal disease, unresected ALL paraspinal or epidural tumors, regardless of other tumor site(s) |
| IV | Any of the above with initial CNS and/or bone marrow involvement |
CNS, central nervous system; GI, gastrointestinal.
Patterns of Tumor Spread
NHL is generally a disseminated disease involving lymph node groups through hematogenous spread, and multiple organs may be involved as well as the bone marrow. Thus, nodal enlargement might be seen at CT in the neck and pelvis, with no abnormality in the chest or abdomen, whereas this distribution would be most unusual for HL. This unpredictability makes a whole body staging technique imperative. However, individual subtypes of NHL are associated with certain patterns of disease. Marked splenomegaly is a feature of splenic marginal zone lymphoma and MCL, the latter often in association with bowel involvement. The constellation of a large anterior mediastinal mass with central venous obstruction and disease in the liver, kidneys, or adrenal glands but little or no nodal disease makes primary mediastinal large B-cell lymphoma (PMBL) the most likely diagnosis, whereas widespread peritoneal disease at presentation, with involvement of the viscera and female genital tract, suggests BL. Certain types of NHL are strongly associated with central nervous system (CNS) or meningeal disease, especially testicular and head and neck lymphoma, and this association may warrant screening of the craniospinal axis or prophylactic intrathecal therapy. With disease progression, nodal lymphoma may spread to involve adjacent structures. In the retroperitoneum, this can affect the paravertebral and paraspinal structures with resultant neural compression. In the mesentery, spread into adjacent bowel loops is common, causing displacement, encasement, or compression. As disease advances, peritoneal involvement can occur, radiologically indistinguishable from peritoneal carcinomatosis.
Often, the pattern of disease at CT may suggest the diagnosis. Thus, involvement of cervical lymph nodes and the tissues of Waldeyer’s ring suggests NHL rather than HL. Nodal enlargement in the anterior and middle mediastinum suggests HL, whereas disease in the mesentery, with or without concomitant bowel involvement, strongly favors NHL (see Table 29-3).
Imaging Techniques
Nodal Disease
Cross-sectional Imaging
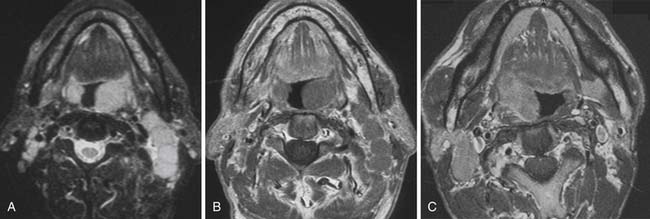
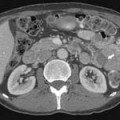
For some time, CT has been the modality of choice for the staging and follow-up of lymphoma. It enables localization of the most appropriate lesion for consideration of percutaneous image-guided biopsy. Ultrasound has limited value in staging. Involved lymph nodes have nonspecific appearances, although the pattern of nodal vascular perfusion on power Doppler sonography may suggest the diagnosis. The main value of ultrasound is in providing image guidance for biopsy. Although magnetic resonance imaging (MRI) is as accurate as CT in detecting lymph node enlargement, its role is essentially adjunctive. As with CT and ultrasound, involved lymph nodes cannot be diagnosed other than by size criteria (Figure 29-2). Advances in scanner technology including high–field strength magnets permit whole body MRI for staging lymphoma. Whole body diffusion-weighted imaging of lymphoma may have a role in the differentiation of lymphoma from other causes of malignant nodal enlargement.15
Functional Imaging with Positron-Emission Tomography (PET) and PET/CT
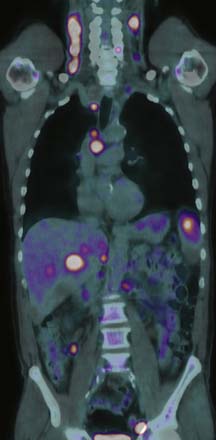
Detection of disease in normal-sized nodes is not possible with cross-sectional imaging, nor is it possible to differentiate between nodal enlargement secondary to lymphoma or reactive hyperplasia. Functional radioisotope studies permit this distinction. Gallium-67 (67Ga) citrate has largely been replaced by positron-emission tomography (PET) using 2-[18F]fluoro-2-deoxy-D-glucose (FDG-PET). In most lymphomas, increased glucose metabolism results in increased cellular uptake of FDG with an accuracy comparable with or better than CT for the detection of nodal and extranodal disease.16,17 In NHL, staging FDG-PET gives important information by indicating tumor burden as well as the presence of extranodal disease (Figure 29-3). Most NHLs show increased uptake, the exceptions being some MALT, cutaneous, and small lymphocytic lymphomas. PET/CT allows accurate co-localization of morphologic abnormalities and their associated functional changes. Debate continues as to whether it is always necessary to carry out a full diagnostic CT scan as part of the PET/CT study or whether a low-dose CT for the purposes of attenuation correction and anatomic correlation is sufficient.18
Key Points Nodal imaging: general
• Recognition of nodal disease with CT and MRI depends on size criteria alone.
• CT and MRI have equal sensitivity below the diaphragm.
• Ultrasound has a role in problem-solving and guidance for biopsy.
• FDG-PET/CT may enable identification of disease in normal-sized lymph nodes.
• Nodal disease in the lymphomas may involve any anatomic lymph node site.
Neck
Cervical lymph node enlargement is seen in 60% to 80% of patients with HL at presentation. It typically involves the internal jugular chain of nodes initially, with further spread to the spinal accessory and transverse cervical chains. In NHL, the pattern of involvement is more haphazard than in HL (see Figure 29-2). Lymph nodes greater than 1 cm in short-axis diameter (SAD) are generally considered enlarged. Enhancement after administration of contrast medium is usually mild to moderate, and central necrosis within a lymph node is rare. MRI may be particularly useful for defining the extent of lymphomatous masses in the lower neck and supraclavicular fossa.
Thorax
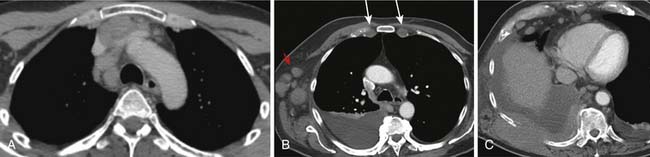
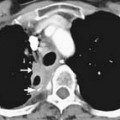
Intrathoracic nodes are involved at presentation in 60% to 85% of patients with HL and 25% to 40% of patients with NHL. Almost all patients with nodular sclerosing HL have disease in the anterior mediastinum. Nodal involvement in mediastinal presentations includes prevascular and paratracheal (84%; Figure 29-4A), hilar (28%), subcarinal (22%), and other sites (~5%), including aortoplumonary, anterior diaphragmatic, and internal mammary (see Figure 29-4B and C).19 In NHL, the incidence varies, but may include superior mediastinal (34%), hilar (9%), subcarinal (13%), and other sites (≤10%).20
The majority of cases of HL show enlargement of two or more nodal groups, whereas only one nodal group is involved in up to half of the cases of NHL. Hilar nodal enlargement is rare without associated mediastinal involvement, particularly in HL. Although nodes in the internal mammary chains and paracardiac regions are rarely involved at presentation, they become important as sites of recurrence because they may not be included in conventional radiation fields (see Figure 29-4C). It is important to review these sites because minimally enlarged nodes are easily overlooked.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree