Abstract
In this chapter, we will review and summarize the relevant literature that studied the effects of delayed cord clamping (DCC) and umbilical cord milking (UCM) in the delivery room, on hemodynamics measurements in the first hours after birth, and on blood volume measurements. Finally, we will discuss outstanding questions and future directions of DCC and UCM.
Keywords
cut umbilical cord, delayed cord clamping, hemodynamics, immediate cord clamping, intraventricular hemorrhage, transitional circulation, umbilical cord milking
- •
Both delayed cord clamping and umbilical cord milking provide a placental transfusion at birth.
- •
An important benefit aside from volume may be the stabilization of the transitional circulation.
- •
There is a good physiologic rationale for delaying umbilical cord clamping until after the infant begins to breathe.
- •
Umbilical cord milking may be superior at providing a placental transfusion at cesarean section and may provide a transfusion more quickly in nonbreathing infants but this needs more study.
Delayed cord clamping (DCC) and umbilical cord milking (UCM) are two techniques that provide placental transfusion to the newborn infant. Increasing fetal hemoglobin and blood volume by placental transfusion is an extremely effective method of enhancing arterial oxygen content, increasing cardiac output, and improving oxygen delivery to the tissues. Placental transfusion is the transfer of residual placental blood to the infant during the first few minutes after delivery. DCC is the practice of waiting to clamp the umbilical cord after birth for at least 30 seconds or longer. Studies have shown that DCC benefits in preterm infants including improved hemodynamics, less blood transfusions, lower rates of intraventricular hemorrhage, and necrotizing enterocolitis, as well as improved motor function at 18 to 22 months of age. Term infants receiving DCC have higher hemoglobin levels at 24 hours after birth and improved iron stores at 3 to 6 months without an increase in reported maternal morbidities. In resource-limited settings, there is observational evidence to suggest that mortality is reduced if umbilical cord clamping (UCC) occurs after the initiation of spontaneous respiration. International recommendations advocate for a delay in UCC for 30 seconds to more than 60 seconds after birth if the infant is vigorous.
It is primarily believed that, after delivery, the major recipient of placental blood is the pulmonary bed. Normally, as the infant initiates spontaneous breathing and establishes lung aeration, the pulmonary blood vessels dilate and the infant will draw blood from the placenta into the dilated pulmonary blood vessels. If infants do not breathe at birth, guidelines recommend immediately clamping the umbilical cord and moving the infant to a resuscitation platform in order to provide positive pressure ventilation (PPV). In the largest randomized clinical trial investigating delivery room respiratory support in infants less than 29 weeks’ gestation at birth, over 60% received PPV. This outcome suggested that many infants would receive immediate cord clamping prior to inflation of the lungs.
UCM is a procedure in which the clinician milks or pushes the blood in the umbilical cord from the placenta to the infant. There are two established techniques of UCM that have been described in the literature. One method is “intact umbilical cord milking.” In this technique, the clinician milks 20 cm of the umbilical cord over 1 to 2 seconds, and releases the umbilical cord after each milk to allow the cord to refill with blood. This process is repeated 2 to 4 times prior to UCC. Another UCM technique, called “cut-umbilical cord milking,” is clamping the umbilical cord close to the placenta and milking the residual volume of blood in the umbilical cord after cord clamping. Due to insufficient evidence, international recommendations currently discourage the use of UCM outside of clinical studies.
The primary advantage of UCM over DCC is the rapid blood transfer from the placenta to the infant immediately after birth without interfering with the evaluation and the resuscitation of the newborn. In several trials, authors have concluded that UCM appears to confer the same benefits as DCC. In addition, UCM may be a more effective method to transfer blood during cesarean deliveries because the uterus is not vigorously contracting.
In this chapter, we will review the relevant literature regarding the effects of DCC and UCM in the delivery room on hemodynamics measurements in the first hours after birth, and on blood volume measurements. Finally, we will discuss outstanding questions and future directions of DCC and UCM.
Transitional Physiology and Animal Studies of Delayed Cord Clamping and Umbilical Cord Milking
Human studies have a limited ability to accurately measure physiologic changes immediately after delivery. Animal models can use invasive monitoring prior to delivery to study the effects of umbilical cord management strategies, specifically to understand the effects on cerebral and pulmonary blood flow.
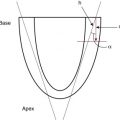
In the fetal phase, the placenta performs the function of gas exchange, providing the fetus with oxygen and eliminating carbon dioxide. The lungs are filled with liquid secreted by the lungs and pulmonary blood flow is low. The umbilical circulation, via the umbilical vein, ductus venosus, and foramen ovale, provides the majority of blood flow to the left ventricle. The placental circulation is a low-resistance pathway that receives up to 50% of the fetal cardiac output. UCC dramatically affects the neonatal circulation by increasing peripheral resistance (afterload), as the low resistance placenta pathway is removed, and by the loss of umbilical venous supply to the left ventricle (preload). As the infant initiates breathing and the lungs aerate, pulmonary blood flow increases, replacing the umbilical venous flow to supply the left ventricle and providing adequate preload. In theory, increasing pulmonary blood flow and ensuring a pathway for a sustained left ventricular preload before UCC would better prepare the infant for the hemodynamic changes of UCC ( Fig. 5.1 ).
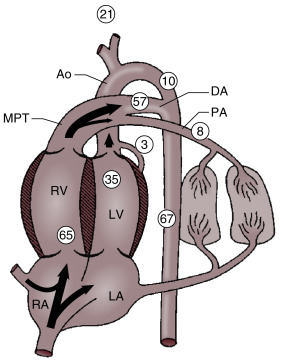
Animal models have shown benefits of initiating ventilation to increase pulmonary blood flow prior to UCC in preterm, anesthetized newborn lambs. Newborn lambs at 126 days’ gestational age (equivalent to ∼26 weeks in humans) with UCC prior to ventilation had dangerous swings in cerebral blood flow, arterial blood pressure, heart rate, and cerebral oxygenation. Lambs that received ventilation prior to UCC had a much smoother transition to ex utero life, including attenuated changes in cerebral perfusion and blood pressure and increased levels of oxygenation. A major limitation is that the fetal lambs in these studies were under anesthesia and paralyzed without the ability to breathe spontaneously. In addition, lambs had their lung fluid drained and in some cases received a 20-second sustained inflation breath prior to being placed on a ventilator. Whereas this model provides important information regarding hemodynamics during placental transfusion, the model does not provide answers in regards to whether ventilation is beneficial or even required during DCC in premature newborns.
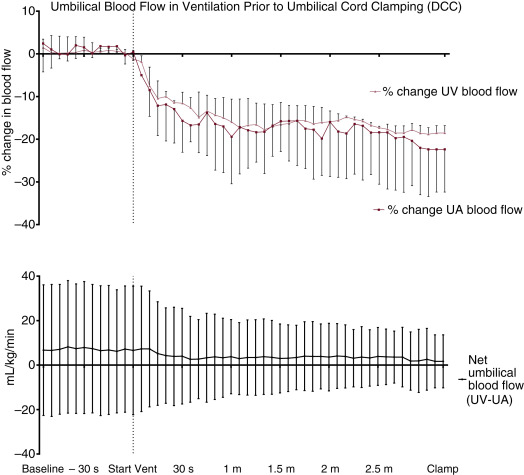
Human studies have suggested that gravity affects the amount of placental transfusion at vaginal birth. Holding the neonate high above the placenta (head 40 to 60 cm above) decreases placental transfusion similar to immediate cord clamping (ICC). A recent study found no difference in infant weights after DCC for 2 minutes with infants placed on the maternal abdomen versus at the introitus. However, total weight gain was half of what was previously found, indicating that 2 minutes may not be enough time for a full placental transfusion for the term infant. Mercer et al. found that term infants placed on the maternal abdomen immediately after birth who were assigned to DCC for 5 minutes received a significantly larger placental transfusion than those with a 2-minute delay. However, the same may not be true for cesarean section (C/S). The effect of gravity on umbilical blood flow during DCC was investigated by measuring umbilical venous and arterial blood flow using ultrasonic flow probes and biotin labeled blood to measure placental transfusion volumes in preterm fetal lambs delivered by C/S. Anesthetized fetal lambs were placed 10 cm above and 10 cm below the ewe during DCC and received subsequent mechanical ventilation prior to UCC. The onset of mechanical ventilation resulted in a decrease in both umbilical venous and arterial flow. The decrease in umbilical arterial and venous blood flow was proportional; therefore the net flow of blood to the fetal lamb did not change. There was no observed increase in blood volume during the period of DCC, therefore no placental transfusion was detected. In addition, the position of the fetal lamb in relation to the ewe did not affect the net flow of umbilical blood to the fetal lamb. Further animal studies are needed to explore physiologic changes during spontaneous breathing after birth and vaginal delivery.
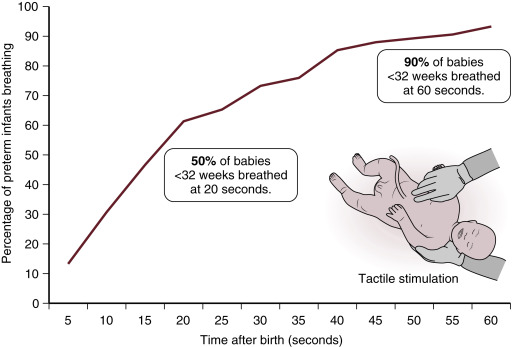
Improved physiologic stability may play an important role in the benefits of DCC observed following C/S. In the animal model described earlier, ventilation prior to UCC increased pulmonary blood flow and resulted in less fluctuations in blood pressure, cerebral blood flow, and cerebral oxygenation; however, significant placental transfusion resulting in increased blood volume was not observed ( Fig. 5.2 ). A possible explanation for these findings is the lack of spontaneous breathing in the anesthetized animals. Spontaneous breathing is commonly observed after delivery even in the most premature human infants. It may also stimulate placental transfusion by opening pulmonary capillary beds. In a recent trial of premature infants randomized to assisted ventilation or tactile stimulation there was no difference in resuscitation interventions, transitional hemodynamics, or neonatal outcomes. However, over 90% of premature infants had spontaneous ventilation whether they were provided with tactile stimulation alone or assisted ventilation ( Fig. 5.3 ). It may be that a mechanism of benefit of DCC is allowing time for spontaneous breathing prior to the clamping of the cord and thus, by maintaining left ventricular preload, ensuring an unperturbed hemodynamic transition. Further clinical trials are needed to better determine whether assisted ventilation (with continuous positive airway pressure [CPAP] and positive pressure ventilation [PPV]) provides benefit during a placental transfusion.
Cardiovascular Effects of Delayed Cord Clamping and Umbilical Cord Milking in the Delivery Room
Despite the difficulties in obtaining accurate physiologic data immediately after birth of human infants, there are a few studies which have investigated the cardiovascular adaptation in newborns during DCC. In a cohort of healthy term, vaginally-delivered infants, arterial and venous umbilical blood flow was measured using Doppler ultrasound starting 30 seconds after birth until the umbilical cord was clamped. Several different patterns of umbilical cord blood flow were observed, emphasizing that the physiology of umbilical cord blood flow is complex. The initiation of breathing appeared to promote venous flow to the newborn; however, crying often caused a reversal of flow. Arterial blood flow was observed to continue after umbilical cord pulsations ceased and umbilical arterial and venous blood flow often stopped at different times. The potential volume of the placental transfusion is increased if there is differential constriction of the umbilical arteries prior to the umbilical vein. In another cohort of healthy term infants, continuously monitored cardiac output was measured via electrical impedance during DCC starting 90 seconds after birth. Every minute of postnatal life that the cord was kept unclamped, the stroke volume increased and cardiac output increased 13% compared to baseline. The increase in stroke volume and cardiac output was observed even after umbilical cord pulsation ceased. Again, these findings underscore the importance of allowing for a smooth change from placental to pulmonary blood flow supplying the left ventricular preload during the immediate transitional period.
In animal studies, DCC has been shown to improve oxygen saturations (SpO 2 ) and attenuate the swings in blood pressure and cerebral blood flow seen in ICC. After birth, if the infant is still connected to a functioning placenta that continues to provide gas exchange and left ventricular preload, one might expect infants with DCC to have higher heart rates and higher SpO 2. A few small studies have reported the heart rate and SpO 2 immediately after birth, in preterm and term infants who receive DCC. Compared with normative data, the studies have conflicting results of the effects of DCC ( Table 5.1 ). Winter et al. reported all infants had a heart rate greater than 100 BPM at 60 seconds in a small pilot trial in which respiratory support could be provided during DCC in infants less than 32 weeks. Finally, there is very little data on the effects of UCM on SpO 2 immediately after birth.
| Study | N | Time of Cord Clamping (s) | GA or BW | HR@1 min, BPM | SpO 2 @1 min, % |
|---|---|---|---|---|---|
| Dawson, 2010 | 468 | <20 | 38 weeks (25–42) | 96 (65–127) | 66 (55–75) |
| Linde, 2016 a | 55 | 44 (29–59) | 3100 ± 428 g | 149 ± 33 | NA |
| Katheria, 2016 | 150 | 64 ± 9 | 28 ± 2 weeks | 115 ± 32 | NA |
| Katheria, 2015 | 20 | 318 ± 32 | 39 ± 1 weeks | 176 ± 15 | NA |
| Smit, 2014 | 109 | 300 (180–420) | 40 weeks (37–42) | 61 (42–146) | 78 (67–87) |
a Gestational age was not reported in this study; heart rate reported at 40 seconds.
A small, randomized, controlled trial showed that heart rate and SpO 2 were higher immediately after birth, requiring lower amounts of inspired oxygen (FiO 2 ) and mean airway pressure, in preterm infants receiving intact UCM versus infants receiving ICC. This suggests that cord milking may enhance early pulmonary blood flow and decrease pulmonary pressures. The decreased need for oxygen and mean airway pressure coincides with the finding that these infants also had a lower incidence of oxygen requirement at 36 weeks’ postmenstrual age. There are no published results of the physiologic effects of cut-UCM in the delivery room.
Hemodynamic Measurements in the First Hours After Birth Following Delayed Cord Clamping and Umbilical Cord Milking
Studies have demonstrated that low systemic blood flow during the first 24 hours after birth increases the risk of peri/intraventricular hemorrhage (P/IVH), neurodevelopmental impairment, and death in extremely preterm infants. Assessment of systemic blood flow, such as superior vena cava (SVC) flow, potentially provide a more accurate assessment of cardiovascular adequacy than blood pressure alone. In trials, cardiovascular support with vasopressor-inotropes or inotropes and fluid boluses may increase low systemic blood flow but these interventions have not prevented adverse consequences such as the development of P/IVH nor have they improved neurodevelopmental outcomes (see Chapter 1 , Chapter 2 , Chapter 3 , 6 and 7). As suggested earlier, DCC and UCM may aid in improving immediate hemodynamic transition and thus circulatory stability and also increase circulating blood volume after birth. Consequently, there is interest in studying umbilical cord management strategies and their role in prevention of low systemic blood flow states and subsequently decreasing the risk of P/IVH.
Recent studies measuring systemic blood flow by echocardiography that compare DCC to ICC have conflicting results ( Table 5.2 ). In a small study by Sommers et al., DCC improved SVC flow compared to ICC. However, in a much larger multicenter randomized controlled trial, DCC failed to show any improvement in SVC flow and infants randomized to DCC demonstrated lower right ventricular output. Unfortunately, 22% of the infants allocated to the DCC arm had cord clamping prior to the targeted clamping at 1 minute mainly due to the obstetricians being uncomfortable with waiting when infants were less vigorous. However, it is in these very infants where DCC might be most advantageous. Several trials have shown that DCC in preterm infants leads to higher blood pressure in the first hours after birth and less treatment for low blood pressure, compared with infants receiving ICC.