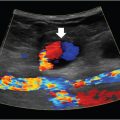
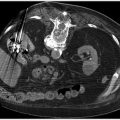
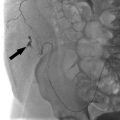
6 Hepatobiliary Interventional radiologists care for patients with both acute and chronic hepatobiliary diseases. Patients with acute hepatobiliary disease most commonly present with right upper quadrant (RUQ) pain, and are diagnosed with a combination of labs and imaging. Patients with chronic disease may be asymptomatic, and only diagnosed after incidental imaging findings demonstrate morphological changes in the liver. In either case, identifying the pattern of liver function tests (LFTs) is often a good first step when approaching disease of the hepatobiliary system. Abnormal LFTs should be interpreted in conjunction with the patient’s history and physical exam. This should narrow the differential prior to obtaining imaging. It is not necessary for you to understand the full differential and work-up, but a basic understanding of certain LFT patterns is useful. Elevated transaminases are often an indication of hepatocyte damage, and the degree of elevation is an important distinction. Numbers in the thousands are seen with viral hepatitis, drug-related or ischemic damage. Numbers in the hundreds may be due to chronic hepatitis, other infections, or hepatic congestion related to heart failure. Mild elevations might be seen with alcoholic liver disease. While alanine aminotransferase (ALT) is specific to hepatocytes, aspartate aminotransferase (AST) is less so, and can be elevated in rhabdomyolysis or other muscle disorders. If liver disease is suspected and labs show a significantly elevated alkaline phosphatase (ALP), cholestasis or biliary obstruction should be considered. As with AST, ALP is nonspecific, and an isolated elevation in ALP can be related to a number of different diseases. However, elevation of ALP in conjunction with elevation in gamma-glutamyl transferase (GGT) increases the specificity for liver disease. A direct hyperbilirubinemia suggests that hepatocytes are functioning but that the liver is not able to excrete normally, possibly due to obstruction. Indirect hyperbilirubinemia is more consistent with hemolysis or intrinsic hepatocyte dysfunction. Certain labs can also indicate the overall health of the liver. If the liver is significantly damaged, you may note signs of compromised synthetic function. Elevated prothrombin time (PT)/international normalized ratio (INR) and low albumin can be seen, but other confounding factors such as malnutrition, malabsorption, or warfarin use need to be ruled out to be certain synthetic dysfunction is to blame. The liver consists of three functional lobes: the right, left, and caudate. The middle hepatic vein separates the left and right lobes. The falciform ligament, portal and remaining hepatic veins further subdivide the right and left lobes into eight functional segments, known as the Couinaud’s classification system (▸Fig. 6.1). Each segment has its own arterial supply, venous and biliary drainage. The arterial supply to the liver originates from the celiac axis via the common hepatic artery, though this represents only a portion of the blood supply. Greater than 75% comes from the portal vein. The portal venous system includes veins that drain blood from the GI tract, spleen, and pancreas. The main portal vein is formed by the superior mesenteric and splenic veins. It enters the liver at the porta hepatis, where it bifurcates into the left and right hepatic lobes. Fig. 6.1 Hepatic anatomy showing the liver segments and their relationship with the hepatic veins, hepatic arteries, and bile ducts. (Source: 9 Normal Anatomy and Variants. In: Beek E, Van Rijn R, eds. Diagnostic Pediatric Ultrasound. 1st Edition. Thieme; 2015.) The hepatic veins, as part of the systemic circulation, allow passage of blood from the liver parenchyma to the IVC at the confluence of the right, middle, and left hepatic veins (▸Fig. 6.1). The caudate lobe drains directly into the IVC (which is why it is typically spared in disease processes such as Budd–Chiari syndrome). There are a number of anastomoses between the systemic and portal venous systems, which will be important to understand for our discussion of portal hypertension. The intrahepatic biliary system is comprised of the right and left hepatic ducts, which run in parallel to the portal veins, and join to form the common hepatic duct. The caudate lobe biliary drainage is variable, but typically occurs through ducts that join to both the left and right hepatic ducts. The left and right hepatic ducts join to become the common hepatic duct, which joins the cystic duct extrahepatically to become the common bile duct (CBD). The normal physiology of the biliary system is neurohormonally regulated, alternating storage and passage of bile during fasting and digestive states, respectively. When this flow is disrupted, it’s thought that rising concentrations of cholesterol in the bile induces an inflammatory response along the bile duct epithelial lining. Inflammation is often, but not always, associated with some form of mechanical obstruction within the biliary tree. Patients with biliary disease present differently depending on a number of factors. The location of the obstruction/inflammation within the biliary tree determines the degree of laboratory derangements and presence or absence of jaundice. Pain is typically a consequence of how rapidly the problem arises and may be absent in chronic cases. The acuity of the problem, and hence the approach to management, is determined by the degree of inflammation and sometimes the presence of a superimposed infectious process. From the IR perspective, biliary disease can be broken down into pathology of the gallbladder, CBD, or hepatic ducts. Acute cholecystitis is most often the result of gallstone disease, although it can also occur in patients without stones (acalculous cholecystitis). A stone that obstructs the cystic duct will result in an inflamed gallbladder. Patients classically present with RUQ pain, low-grade fever, nausea, vomiting, and elevated white count. The pain may be colicky at first, but then becomes constant. LFTs are typically normal or only mildly elevated. Significant LFT abnormalities should be a clue that the problem resides elsewhere in the biliary system. Patients with a presentation suggestive of acute cholecystitis will initially undergo a RUQ ultrasound. Positive findings include a distended gallbladder with cholelithiasis, wall thickening, pericholecystic fluid, and a sonographic Murphy’s sign (pain when applying pressure with the ultrasound probe directly over the gallbladder). Wall thickening is the most sensitive sign, but it is not specific. When ultrasound findings are indeterminate, a nuclear medicine HIDA scan can be used. The HIDA scan is the most accurate imaging modality for diagnosing cholecystitis; a positive study will demonstrate lack of radiotracer filling of the gallbladder, which indicates cystic duct obstruction. Contrast-enhanced CT or MRI is more sensitive for the detection of gangrenous cholecystitis, which is a severe complication that results from ischemia of the gallbladder wall. The combination of a positive physical exam, systemic signs of inflammation, and at least one characteristic imaging finding is required to make a definitive diagnosis of acute cholecystitis. Once diagnosed, the patient is made NPO, analgesics and antibiotics are administered, and general surgery is consulted. For symptomatic patients who are healthy enough to undergo surgery, laparoscopic cholecystectomy (lap chole) is considered the treatment of choice. In addition to symptomatic relief, prompt surgical management can prevent complications of acute cholecystitis such as gallbladder rupture and sepsis. Surgery should ideally be performed within the first day of hospitalization, as delaying it has been shown to increase the incidence of complications and conversion to open cholecystectomy. Those who are too sick to undergo surgery may benefit from placement of a percutaneous cholecystostomy tube (PCT) by IR (Procedure Box 6.1) (▸Fig. 6.2). The purpose of the tube is to drain the infected fluid and decrease gallbladder inflammation. Although it is not as definitive of a treatment as surgery, it can achieve adequate symptom control and reduce the risk of complications, while allowing the inflammatory process to cool down. There are few contraindications to PCT placement, which is fortuitous for very sick patients. Ascites has historically been considered a relative contraindication out of concern that it could prevent tract formation for the tube, but more recently studies have disproven this. Determining who should undergo surgery versus PCT is not always clear-cut. Recommendations set forth by the Tokyo guidelines, updated most recently in 2018, offer a grading system for management based on a number of patient risk factors (▸Table 6.1). Grade I (mild) acute cholecystitis patients should undergo a lap chole, provided they are healthy enough to undergo surgery from a comorbidity standpoint. Those who are not healthy enough for surgery are treated conservatively with antibiotics. Surgery is considered if their status changes favorably. Grade II (moderate) patients can undergo a lap chole if they are healthy enough and they are treated at an advanced surgical center. Otherwise, biliary drainage with PCT should be considered when the patient does not respond to initial medical management. When surgery is pursued, the surgeon should be prepared to convert to an open cholecystectomy or perform a subtotal cholecystectomy, if required. Grade III (severe) patients have evidence of organ dysfunction and have historically been considered noncandidates for surgery. The updated guidelines suggest that renal dysfunction and cardiovascular dysfunction are more favorable than the other types of organ failure, and therefore may not preclude surgery if there is appropriate initial medical management and the patient is treated at an advanced surgical center. However, the majority of grade III patients are better off treated with PCT placement. Cholecystostomy placement is most commonly performed for drainage of the gallbladder in the setting of acute cholecystitis in nonsurgical candidates. Percutaneous gallbladder access may be obtained through a transhepatic or transperitoneal approach. A transhepatic approach is often preferred because of greater catheter stability, quicker tract maturation, decreased incidence of bile peritonitis, and less chance of bowel injury. Transperitoneal access is typically reserved for patients with diffuse liver disease, uncorrectable coagulopathy, or a pendulous gallbladder positioned far from the liver surface. The gallbladder is usually accessed using a 21- or 22-gauge needle, and intraluminal access confirmed with 5 cc of contrast; a large contrast dose should be avoided as this could potentiate sepsis. Once a guidewire is placed into the gallbladder, the cholecystostomy drain can be advanced over the wire. In select patients and in the hands of an experienced operator, direct gallbladder access using a sharp stylet and trocar technique can also be performed. Postprocedurally, the catheter should be gently flushed every 24 hours. The catheter tract matures in about 4 to 6 weeks; long-term catheters should be changed every 3 months. The drain can be pulled if there is confirmed resolution of the cholecystitis and evidence of a patent cystic duct, confirmed by a tube cholecystostogram. Sometimes the drain remains in place until the patient goes to the operating room for an interval cholecystectomy and is removed at that time. Fig. 6.2 An 8.5-Fr pigtail cholecystostomy tube placed by a trans hepatic approach for a patient with acute cholecystitis who was a poor surgical candidate. Table 6.1 Tokyo guidelines severity grading for acute cholecystitis
6.1 General Anatomical Principles
6.2 Biliary Disease
Gallbladder Disease
Grade | Criteria |
I | Healthy patient without organ dysfunction |
II | WBC > 18,000 |
III | Cardiovascular dysfunction, hypotension requires pressors |
Abbreviations: RUQ, right upper quadrant pain; WBC, white blood cells. | |
For those who undergo PCT placement, a cholecystectomy can be performed on an elective basis once inflammation has subsided and the patient is healthy enough for surgery. No randomized controlled trials have yet been performed to determine the ideal timing of cholecystectomy after PCT, but a few observational studies have suggested that early surgery after percutaneous gallbladder drainage is associated with a higher incidence of complications. There is no consensus on optimal timing for surgery after PCT, but that may change as more data become available.
The Tokyo guidelines grading system is validated by a multitude of retrospective studies which have identified factors associated with higher operative risk in acute cholecystitis patients. Even with all of these studies, it is difficult to directly compare outcomes between emergent lap chole and percutaneous drainage (with or without subsequent cholecystectomy), as there is an inherent selection bias for sicker patients receiving the latter. The CHOCOLATE trial is a multicenter prospective study that seeks to sort this out by randomizing management of high-risk acute cholecystitis patients to either lap chole or PCT. When published, the results of this large prospective study may redefine how PCT fits into the treatment algorithm for acute cholecystitis.
Acalculous cholecystitis represents a special circumstance in which PCT may be beneficial. In contrast to calculous cholecystitis, these are usually inpatients who are severely septic with no known source. As part of the sepsis work-up, a RUQ ultrasound might be performed if there is suspicion for a biliary etiology. With acalculous cholecystitis, the gallbladder will show signs of inflammation without identifiable stones. Some of these patients will meet some, but not all, of the diagnostic criteria for acute cholecystitis. If a PCT is placed for one of these patients and he or she improves, it supports acalculous cholecystitis being the source of sepsis. The drained fluid can be cultured for the purpose of tailoring antibiotics.
When gallstones cause repeated bouts of subacute cholecystitis, chronic cholecystitis can result. Radiographically, the gallbladder is fibrotic, shrunken, and filled with gallstones. The treatment for chronic cholecystitis is cholecystectomy, especially if a porcelain gallbladder is present (as this can also be seen in gallbladder carcinoma). IR is not typically involved in the care of patients with chronic cholecystitis unless they have an acute flare and have an indication for PCT.
Bile Duct Disease
Cholecystitis affects the gallbladder and cystic duct, while in most circumstances the remainder of the biliary tree remains relatively unaffected, allowing unimpeded flow of bile from the liver to the duodenum. When obstruction and inflammation affect the CBD or hepatic ducts, bile no longer flows freely. It backs up within the liver, causing hepatic dysfunction and increased bile components entering the systemic circulation. In these patients, labs will show a direct bilirubinemia, and the patient may present with scleral icterus, jaundice, and clay-colored stools.
Acute obstruction of the CBD occurs in choledocholithiasis. With passage of a gallstone into the CBD resulting in obstruction, the pathophysiology of choledocholithiasis is similar to cholecystitis, stemming from the inflammatory reaction of cholestasis. Patients have RUQ pain, nausea, vomiting, and mild fever, but additionally have bilirubinemia. Lab values may also identify elevated transaminases, ALP, and GGT.
Chronic obstruction of the biliary ampulla or bile ducts can be due to a number of benign etiologies, including anastomotic strictures, or due to malignant etiologies such as pancreatic cancer or cholangiocarcinoma (▸Table 6.2). As these are relatively slow to develop, the patient commonly presents with painless jaundice. When there is complete occlusion of the CBD, the gallbladder becomes distended and may be palpable on exam (Courvoisier’s sign).
Biliary obstruction, regardless of the etiology or chronicity, is a risk factor for ascending cholangitis. Disruption of the normal flow of bile can permit retrograde migration of bacteria from the small bowel into the biliary tree. Once inside the bile duct, infection can rapidly spread into the bloodstream and result in sepsis. These patients present with jaundice, fever, and RUQ pain (Charcot’s triad), as well as a leukocytosis and eventually hypotension.
Table 6.2 Etiologies of bile duct obstruction
Benign | Malignant |
Trauma | Primary hepatobiliary neoplasm |
Surgery and radiation | Pancreatic neoplasms |
Infection | Gallbladder carcinoma |
Primary sclerosing cholangitis, pseudocyst, chronic pancreatitis | Regional lymph node enlargement |
Ischemia |
|
Mirizzi’s syndrome, gallstones, portal cholangiopathy |
|
When a patient presents with jaundice, before any imaging is ordered, it is important to rule out emergent problems including acute cholangitis, liver failure, or massive hemolysis. These can be life threatening and require immediate attention.
If there is clinical suspicion for biliary obstruction based on history and labs, the preferred initial imaging modality is ultrasound. A dilated CBD on ultrasound is suggestive of a distal obstruction within the duct, and choledocholithiasis is likely if the presentation is acute. Although ultrasound does not always identify the obstructing stone (frequently the case due to overlying bowel gas), the presence of stones in the gallbladder and the finding of a dilated CBD strongly suggests choledocholithiasis. The absence of stones in the gallbladder implies there may be another cause. If the CBD is nondistended, obstruction may be more proximal in the intrahepatic ducts.
Abdominal CT is also commonly used for initial imaging (▸Table 6.3). CT is quick and is not operator dependent. While not ideal, CT can show dilated ducts and/or calcified stones, as well as exclude alternative diagnoses. CT may be preferred in two scenarios: (1) when there is low suspicion for obstruction and a detailed look at the liver is desirable, or (2) when the presentation strongly suggests malignant obstruction and the scan will be better at delineating the mass. Imaging features of a malignant obstruction include a periductal mass, biliary hyperenhancement, wall thickness greater than 1.5 mm, long segment involvement, and asymmetric wall thickening. A smooth, symmetric, or focal stricture is more likely benign.
Table 6.3 Imaging options for biliary obstructions
Diagnostic tool | Advantages | Disadvantages |
RUQ US | Great for evaluating gallbladder pathology, common bile duct, focal liver lesion | Operator-dependent; body habitus and bowel gas can limit evaluation |
CT abdomen | Comprehensive cross-sectional anatomy provided; rapid, widely available, and reproducible | Radiation exposure; does not evaluate gallbladder pathology as well as RUQ US; does not adequately evaluate distal biliary tree |
MRCP | Provides full delineation of the biliary tree | More expensive, time consuming, and susceptible to motion artifact |
ERCP | Provides delineation of the distal biliary tree; can relieve distal obstructions through stenting; can perform sphincterotomy to facilitate stone passage; can biopsy distal lesions | Invasive procedure; risk of pancreatitis, hemorrhage, biliary injury; proximal biliary system can be difficult to opacify |
PTC | Provides full delineation of the biliary tree (proximal and distal); can relieve obstruction through stenting; can provide alternate route for biliary drainage | Invasive procedure, traversing liver capsule with risk of hemorrhage; potential patient discomfort if drain is left in place |
Abbreviations: ERCP, endoscopic retrograde cholangiopancreatography; MRCP, magnetic resonance cholangiopancreatography; PTC, percutaneous transhepatic cholangiography; RUQ US, right upper quadrant ultrasound. | ||
Magnetic resonance cholangiopancreatography (MRCP) is an alternative to ERCP. MRCP avoids the use of radiation and contrast, and does not have the same risks of iatrogenic injury as with ERCP. It can delineate the anatomy of the entire biliary tree, including ducts distal to an obstruction (sometimes impossible with ERCP). Disadvantages include susceptibility to artifacts associated with MRI and a reduced sensitivity for detecting small stones or lesions. MRCP is often chosen over ERCP when the patient is too sick to undergo the procedure, and in other circumstances when there is no anticipated need for an intervention.
Management of Biliary Obstruction
Although the utilization of interventional procedures for biliary obstruction has considerable overlap between benign and malignant etiologies, they really ought to be approached differently, in keeping with the unique management goals of each.
The most common, acute cause of benign biliary obstruction is choledocholithiasis. In a patient with choledocholithiasis, the goal is symptomatic relief, as well as avoidance of the most serious complications: cholangitis and pancreatitis. Some patients may already have a complication at the initial presentation, requiring more emergent treatment.
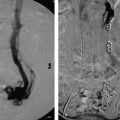
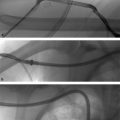
Those that present with acute cholangitis require fluids, broad-spectrum antibiotics, and often ICU admission. After the patient’s status has improved with medical management, typically a day or two into the admission, ERCP can be performed to attempt to remove the obstructing stone. After cannulating the ampulla of Vater, stones can be removed with the use of a snare or balloon. Removing the stone and allowing the decompression of upstream infected bile is the key to achieving source control (much like drainage of an abscess). When ERCP is unavailable, anatomically unfeasible, or has already been attempted and failed, IR can assist with percutaneous transhepatic cholangiography (PTC) and percutaneous transhepatic biliary drainage (PTBD) (Procedure Box 6.2) (▸Fig. 6.3, ▸Fig. 6.4). PTBD in the context of treating acute cholangitis has success rates that approach ERCP, however, the rate of complications is somewhat higher.
Gallstone pancreatitis has a wide spectrum of clinical severity. Supportive care is appropriate for the initial management of all patients, however, several studies have shown a benefit of urgent ERCP to restore biliary patency in severe cases, when there is rapidly uptrending LFTs in the first 48 hours of admission. The procedure has been shown to be safe, even in the setting of acute pancreatitis. Percutaneous biliary drainage is not helpful for these patients because the problem stems from the physical presence of the obstructing stone, rather than biliary stasis. Once the patient is stable, cholecystectomy is necessary to prevent recurrent pancreatitis.
Percutaneous access to the biliary tree is performed for diagnostic cholangiography, biliary drain or stent placement, choledochoscopy, cholangioplasty, and treatment of stones. Biliary access can be safely obtained from a right- or left-sided approach. The right lobe ducts are typically accessed from the right mid-axillary line, usually through the lower intercostal spaces. The left ducts are generally accessed from the epigastrium, below the xyphoid process. It is important to avoid potential interposed stomach or bowel with either approach.
After sterile preparation of the abdomen, a 21- or 22-gauge needle is inserted into the liver using ultrasound or fluoroscopic guidance. Ultrasound guidance can often allow for direct access into a biliary branch, while fluoroscopic approaches require the injection of contrast while withdrawing the needle under fluoroscopic guidance until the biliary tree is opacified. Knowledge of fluoroscopic liver anatomy is critical during these pullback injections, as the veins and arteries are often opacified during this technique. When contrast opacifies the biliary tree under fluoroscopy, a cholangiogram is performed in multiple obliquities and the suitability of the initial access site is determined; if the access is too central, the opacified biliary tree can then be targeted more peripherally under fluoroscopic guidance with a second needle.
After performing cholangiography, a wire is advanced through the needle into the central biliary ducts. The needle is removed and replaced with a transitional dilator which can be disassembled to accommodate a catheter and/or larger guidewire. This facilitates crossing an obstruction and placement of a stiff guidewire for subsequent drain placement or intervention. If the obstruction cannot be crossed, a pigtail drain is placed in the central biliary ducts to allow external drainage; after several weeks of external drainage, inflammation often subsides and strictures or obstructions may be easier to cross. Of note, in the setting of cholangitis, overdistension of the biliary tree with contrast and extensive wire manipulation or intervention should be avoided in the initial access procedure, as this may precipitate biliary sepsis. Serious complications of PTC/PTBD include bleeding, bile leak, and pneumothorax. Arterial hemorrhage can be life threatening and is more likely to occur when the access is too central. Minor hemorrhage may be clinically silent, but more severe hemorrhage can be life threatening and needs to be addressed with an emergent hepatic artery embolization.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree