Male and female combined
Age (years)
34.2 (18–51)
Weight (kg)
74.6 (40–120)
Femoral component size (mm)
49.2 (40–54)
Charnley class
A
17 (37 %)
B
22 (48 %)
C
7 (15 %)
In our series, three different materials were used to replace the femoral head: titanium (11 hips), alumina ceramic (11 hips), and cobalt-chromium (32 hips). The titanium and alumina components were custom-made by Zimmer Inc. (Warsaw, IN) and Kinamed Inc. (Newbury Park, CA), respectively. Two of the CoCr components were femoral shells from THARIES hip resurfacing systems (Zimmer Inc., Warsaw, IN), while the remaining 30 were Conserve® femoral resurfacing components (Wright Medical Technology Inc., Arlington, TN). All components were cemented with the exception of one alumina component that was press fit. A posterior approach was used in the last 37 hips, while the first 17 were resurfaced through a posterolateral approach with a trochanteric osteotomy. The details of the surgical technique used have been previously described [39–41], but it is useful to emphasize the need to completely remove the necrotic bone from the reamed femoral head in order to limit the bone-cement interface to vascularized bone only.
51.3.1.4 Results of Hemiresurfacing
Clinical Scores
The average UCLA hip scores all improved significantly (p < 0.001) between preoperative and last follow-up visits. The pain score increased from 4.8 ± 1.8 to 8.4 ± 1.7, the walking score from 5.8 ± 1.8 to 8.9 ± 1.4, the function score from 5.2 ± 1.6 to 7.9 ± 2.0, and the activity score from 4.2 ± 1.3 to 5.9 ± 1.3. The mean postoperative Harris hip score was 82.3 ± 12.4 (range, 54–100). In this series 13 patients (13 hips, 24 %) reported incomplete pain relief (highest UCLA pain score of 7 or less). We found a weak but significant negative correlation in male patients between weight at the time of surgery and the maximum UCLA pain score reported during the follow-up period (r = −0.38, p = 0.022). Patients weighing 77 kg or less had a mean pain score of 9.0 (range, 5–10) while patients heavier than 77 kg had a mean score of 7.4 (range 5–10). This difference was significant (p = 0.006).
Conversions and Survivorship
Twenty-nine hips have now been revised in this series at a mean time of 100.8 months (range, 3–278 months) most for cartilage wear [27]. There was one each revised for sepsis and enigmatic pain at 3 and 12 months. Most failed hemiresurfacing procedures were revised to conventional stem-type total hip replacements with 4 exceptions: Two hips (2 patients) with ceramic hemiresurfacing components received a metal-on-metal hip resurfacing devices (Conserve®Plus, Wright Medical technology Inc., Arlington, TN) after removal of the alumina femoral component, one hip with a CoCr femoral component had a custom cross-linked PE liner cemented on the acetabular side now 10 years post-op, and one hip with a super finished CoCr component (the current Conserve®Plus device, available in 2 mm increments) was converted to a metal-on-metal resurfacing by inserting the Conserve®Plus porous-coated monoblock acetabular component of the corresponding size. The overall Kaplan-Meier survivorship of this series was 79.3 % (95 % confidence interval 65–88 %) at 5 years, 56.4 % (95 % CI 39–70 %) at 10 years, and 26.3 % (95 % CI 12–43 %) at 15 years. The comparative survivorship curves generated by type of material used revealed no significant difference in favor of any of the materials (logrank test p = 0.6628) as shown in Fig. 51.1. We found no association between survivorship and the following variables: gender, age at surgery, Ficat stage, acetabular cartilage grading, component size, and patient weight at the time of surgery.
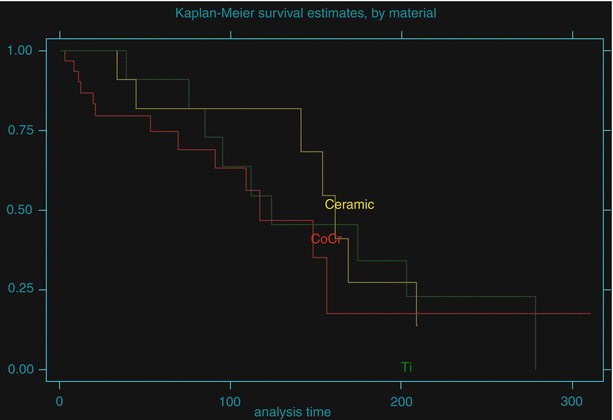

Fig. 51.1
Comparative Kaplan-Meier survivorship curve by femoral head material used in hemiresurfacing procedures between 1981 and 2004. There was no significant superiority of any material (logrank test p = 0.6628)
51.3.1.5 Discussion
The clinical scores showed considerable variability, and this result has also been observed by others [42]. Some patients often report incomplete pain relief during the first few months after the procedure but this typically abates over time due to “accommodation” although these patients rarely become as pain free as those receiving a full replacement. When the acetabular cartilage finally wears out, pain may reappear. However, the longest survivor is a woman with SLE who is now 31 years post-op with no increase in symptoms. Although her joint space has somewhat narrowed, the patient’s UCLA hip scores remain high with 9, 7, 8, and 6 for pain, walking, function and activity, respectively. Weight appears to be related to the magnitude of pain relief, and this was observed particularly in our male patients. Our current recommendation is to reserve this procedure to lighter patients, in particular if the acetabular cartilage has already been somewhat compromised. However, the difficulty to predict the postoperative clinical scores requires that the patient understands the benefit of delaying the implantation of an artificial bearing material. Seven of our patients have been implanted with a hemiresurfacing on one side and a full resurfacing on the contralateral side. Five of these hemiresurfacing have been revised at a mean follow-up time of 59.6 months (range, 11–109) which is less than the mean 100 months of all the revised hips. A possible explanation is that the patients who can compare the hemiresurfacing side with another prosthetic joint are more likely to request a revision because the pain relief is not as good on the hemiresurfacing side.
The survivorship of hemiresurfacing does not match that of other prosthetic treatments (i.e., full resurfacing or conventional THA) but fits within acceptable ranges for a “time-buying” procedure with extremely low morbidity destined to restore the patient’s lifestyle without compromising bone stock in anticipation of an inevitable revision surgery. It was our belief that hard bearing surfaces such as cobalt-chromium or alumina would reduce the friction against the acetabular cartilage and eliminate the metallic debris possibly generated by the relatively soft titanium alloy component (our first hemiresurfacing device). Our comparative survivorship results show that femoral head material has no effect on the durability of the procedure. The advantage of the ceramic is that it can be cracked and removed without loss of bone, and full resurfacing has been successful in 2 patients for over 15 years after such revision. Unfortunately, ceramic components are not now available but would be my choice if still available. With our relatively small series, there has not been an association between survivorship gender, age, or Ficat stage, acetabular cartilage grading, component size, or patient weight, and this illustrates the difficulty to profile the ideal candidate for the procedure. We do believe that the long-term survival of some of these prostheses had to do with how precisely these implants were fitted to the acetabular cartilage in patients with relatively low activity levels.
When reoperation was needed, revision to either full surface or total hip replacement was easy and much like a primary replacement because of bone stock preservation, because of intact intramedullary canals, and because there was no debris-induced granuloma. It is important to note that a regular radiographic follow-up of the patients with hemiresurfacing devices is needed because once the acetabular cartilage is worn, a rapid migration of the femoral head through the acetabulum can occur, leading to bone loss and therefore negating one of the main advantages of hemiresurfacing. The histological response has been very benign with a few macrophages and some metallic debris scattered throughout a predominantly loose connective tissue despite significant burnishing of some titanium alloy components.
At the beginning of the series, in 1981, our indications for hemiresurfacing included patients in their 40s or even 50s or with advanced cartilage defects because there was no alternate conservative prosthetic solution available with demonstrated potential for durability. However, because of the recent success of our metal-on-metal resurfacing series for osteonecrosis [43–45], our indications for hemiresurfacing have narrowed to very young with a well-preserved joint space. We do not include heavy patients anymore, considering that the results of full hip resurfacing are excellent in this patient population [46]. In addition, hemiresurfacing components once available in 1 mm increments for optimal precision fitting to the acetabulum are now only manufactured in 2 mm increments because of cost-efficiency concerns. However, they are finished so that an acetabular component can be inserted, provided there is enough acetabular bone stock.
51.3.1.6 Summary for Hemiresurfacing
From our experience and that of others, we can conclude that it is unrealistic to expect a pain relief and survivorship results comparable to that of full resurfacing or conventional THA in every case. The patients who could benefit from hemiresurfacing are very young and should be fully informed and accept the “time-buying” objectives before undergoing surgery. A surprisingly long durability has been demonstrated in some patients, which may have been favored by low activity levels without participation in sports.
51.3.2 Full Hip Resurfacing
51.3.2.1 Rationale for Full Hip Resurfacing
Primary hip replacement surgery is such a successful procedure that the percent of young patients seeking this type of treatment is increasing every year worldwide [47]. Although the durability has been impressive for some patients, conversion to THR may still be necessary for others. However, full hip resurfacing is a bone-conserving procedure that will provide complete pain relief and preserve both bone quality [48, 49] and quantity [50] for an eventual revision surgery.
51.3.2.2 Indications for Full Hip Resurfacing
Full hip resurfacing should be considered in patients about 25 years of age or over with Ficat III ON or younger with advanced Ficat III or IV ON with extensive cartilage damage. A recent publication showed that the size of the lesion does not alter the survivorship results of hip resurfacing [51] even though previous work with finite element analysis predicted adverse biomechanical effects after resurfacing in presence of large femoral defects [52]. In our series, decision was made from the beginning to include very large lesions as long as most of the cylindrically reamed bone was intact circumferentially. Figure 51.2 shows an example of large defects associated with longstanding osteonecrosis of the femoral head in a patient who received a Conserve®Plus hip resurfacing device.
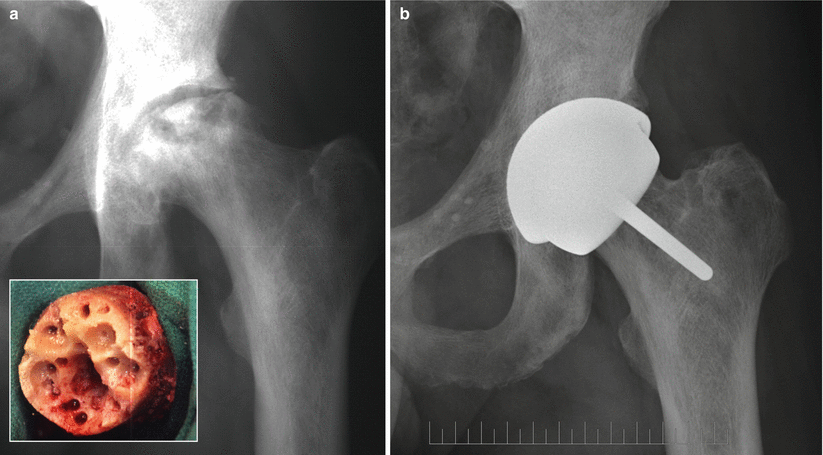

Fig. 51.2
(a) Anteroposterior radiograph of a 46-year-old bus driver with a history of sickle cell disease and Ficat stage IV osteonecrosis of the left hip. The femoral head defects were extensive as shown on the intraoperative photograph of the femoral head after preparation (inset). (b) Twelve years after surgery, the components show perfect interfaces with the bone, and the patient’s UCLA hip scores are 10, 10, 10, and 8 for pain, walking, function, and activity, respectively
51.3.2.3 Methods and Materials
Patient Demographics
From our series of 1,366 hips (1,097 patients), 100 hips (82 patients) received a Conserve®Plus hip resurfacing system (Wright medical Technology, Inc.) for a diagnosis of arthritis secondary to osteonecrosis of the femoral head. The risk factors for the development of ON were distributed as follows:
Steroids 34 (34 %)
Trauma 23 (23 %)
Alcohol 7 (7 %)
Sickle cell disease 1 (1 %)
No dominant risk factor (idiopathic ON) 35 (35 %)
There were 22 hips rated ON Ficat stage III and 78 rated Ficat stage IV.
The average age of the patients was 41.2 (range, 14–64). Most of the patients were male (67/82, 81.2 %).
From this cohort, 36 patients had bilateral disease (44 %). However, excluding the 23 patients with post-trauma (all unilateral), the incidence of bilaterality was 61 %. There were 4 patients with a contralateral hemiresurfacing, 18 with bilateral full metal-on-metal resurfacing (one of them with one device from another manufacturer), 3 with a contralateral conventional THR, and 7 with a contralateral core decompression. Thirty-six hips (36 %) had undergone at least one previous surgery: 21 had a core decompression, 3 had a hemiresurfacing, 9 had been pinned, 2 had previous free vascularized fibula graft, and 1 had a Judet graft.
The demographics of this group of patients are shown in Tables 51.2a and 51.2b.
Table 51.2a
Demographics of the patients operated with full resurfacing for arthritis secondary to osteonecrosis
Male and female combined | |
|---|---|
Age (years) | 41.2 (14–64) |
Weight (kg) | 81.9 (46–116) |
Femoral component size (mm) | 46.7 (36–54) |
Cysts >1 cm | 85 (85 %) |
Charnley class | |
A | 42 (51 %) |
B | 33 (40 %) |
C | 7 (9 %) |
Table 51.2b
Distribution of hip bone quality by femoral defect size
n | % of hips | |
|---|---|---|
Good bone – no defect | 6 | 6 % |
Defects 0–1 cm | 9 | 9 % |
Defects 1–2 cm | 42 | 42 % |
Defects 2–3 cm | 43 | 43 % |
The percentage of large defects was the highest of any etiologic group undergoing metal-on-metal hip resurfacing with only 15 % having none or a defect size of 1 cm or less.
51.3.2.4 Surgical Technique
The surgical technique used in this series for preparation of the femoral head is quite similar to our current technique for hemiresurfacing and has been described in detail in a previous publication [45]. In this series, 55 hips (55 %) were implanted with the femoral metaphyseal stem cemented [53].
51.3.2.5 Results of Full Hip Resurfacing
Clinical Scores
The average UCLA hip scores all improved significantly (p < 0.0001) between preoperative and last follow-up visits. The pain score increased from 3.6 ± 1.5 to 9.3 ± 1.1, the walking score from 5.8 ± 1.6 to 9.5 ± 1.1, the function score from 5.3 ± 1.6 to 9.3 ± 1.4, and the activity score from 4.2 ± 1.4 to 7.0 ± 1.6. The postoperative Harris hip score was 91.9 ± 11.3.
51.3.2.6 Conversions to THR and Survivorship
There were four conversions to THR in this series. Three were consecutive to a loosening of the femoral component at 23, 61, and 85 months (hips #6, #25, and #455) and one secondary to a loosening of the acetabular component at 56 months.
In addition, one patient with poor bone quality who underwent one-stage bilateral procedures required a revision of the acetabular component after this one protruded through the acetabular wall 3 days after surgery. This complication was attributed to over-reaming with new very sharp “bear claw” reamers on the first side of a bilateral simultaneous hip resurfacing.
Using any cause for revision as end point, the Kaplan-Meier survivorship results for this group of patients were the following (Fig. 51.3):
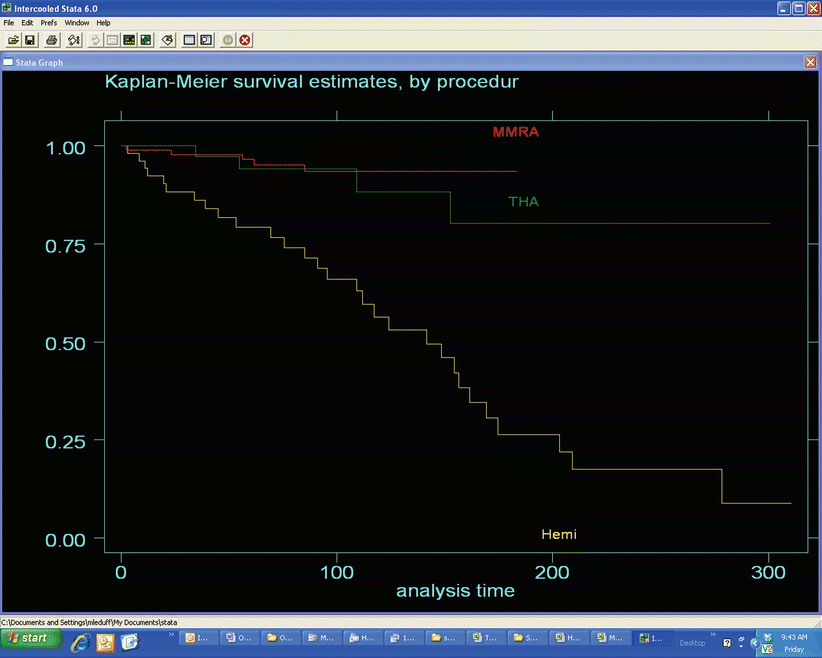

Fig. 51.3
Comparative Kaplan-Meier survivorship curve by procedure used in the treatment of patients with hip osteonecrosis between 1981 and 2012. There was no significant difference between MMRA and THA (logrank test p = 0.3707) while both MMRA and THA were more durable than hemiresurfacing (logrank test p = 0.0001)
5 years: 96.6 % (95 % confidence interval 90–99 %)
10 years: 93.6 % (95 % CI 85–97 %)
We also computed the Kaplan-Meier survivorship results for this group of patients using femoral failure only as end point. This analysis yielded a 98.9 % survivorship at 5 years (95 % CI 92–100 %) and a 95.8 % survivorship at 10 years (95 % CI 87–99 %).
51.3.2.7 Discussion
From the results shown above, it is safe to say that the etiology of osteonecrosis is a good indication for hip resurfacing when performed with optimal technique. Both survivorship and clinical scores match those obtained with idiopathic osteoarthritis [43, 54] and other etiologies [45]. Our survivorship results even surpass those of conventional THR for osteonecrosis listed in the 2012 Australian National registry report (AOANJRR) which shows a 91.8 % survival at 10 years [55]. Two aseptic femoral component loosenings occurred in hips that were implanted with our early surgical technique, before the use of suction, and additional drill holes was implemented [56]. Two hips show radiolucencies around the metaphyseal stem but these have been stable and asymptomatic for over 11 years. There has been no femoral component loosening in the 55 hips with a cemented metaphyseal stem. Since 2005, several studies have suggested that hip resurfacing may be a viable option for patients with femoral head osteonecrosis but none has offered long-term results to this day [43, 44, 57–59]. In the present report, follow-up time ranged from 1 month to 16 years, providing meaningful Kaplan-Meier survival estimates at 10 years.
The question of how much femoral head involvement can be accepted for a resurfacing procedure remains, and both Mont and Revell suggested <33 % of the head as a guideline. Our results confirm with long-term follow-up those of Nakasone [51] and suggest that the extent of femoral head defects should not be a limitation as long as the procedure remains technically possible and the biomechanics of the reconstructed hip are sound.
Summary
Osteonecrosis of the hip presents specific challenges when performing hip resurfacing because of the large defects often present and filled with yellowish, friable necrotic bone. This necrotic bone must be completely removed down to the underlying white hard reparative bone to ensure proper component fixation and durability. Cementing the metaphyseal stem maximizes the fixation area. Our results with the Conserve®Plus device highlight that hip resurfacing should be the treatment of choice for young patients up to the age of 65 with Ficat stage III and IV osteonecrosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree