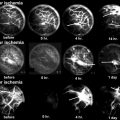
Fig. 58.1
Regional incidence of Perthes’ disease per 100,000 children aged <15 years, 1964–2009. Studies are ordered by latitude. CI confidence interval, IR incidence rate, UK United Kingdom, US United States (Reproduced with permission from Perry et al. [7])
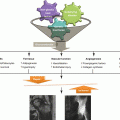
Within countries there is also a marked variation in incidence. This has most clearly been demonstrated in the UK, using a large database of general practice data enumerating 8 % of the population [8]. A profound North–south gradient was identified, with Perthes’ disease rates in the North more than double those in the South, with graduated rates in between (Fig. 58.2).
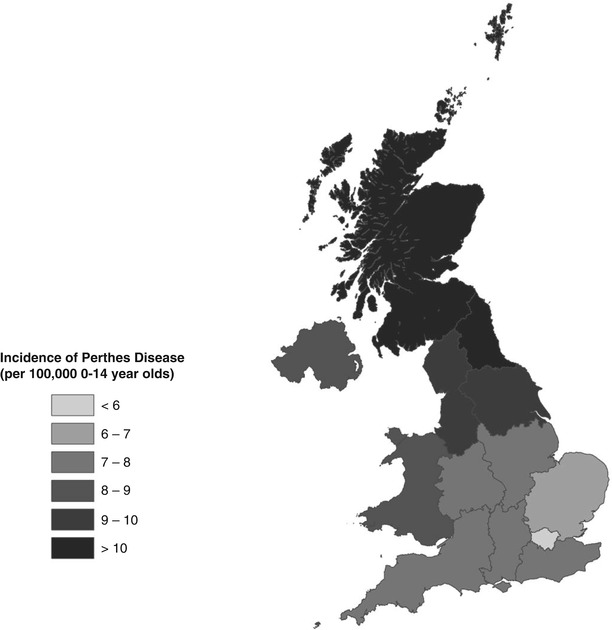

Fig. 58.2
Geographic illustration of the incidence of Perthes’ disease by English Strategic Health Authority, Wales, Scotland, and Northern Ireland (Reproduced with permission from Perry et al. [8], Wiley and Sons)
Even when looking at the lowest levels of UK geography (the Super Output Area (SOA), each unit of which encompasses around 1,500 individuals), rates of Perthes’ disease have varied significantly within towns and cities [9]. In fact, all levels of UK administrative geography demonstrate large differences in Perthes’ disease incidence rates between areas. Similar variations in incidence have also been demonstrated within other countries, such as India and Norway [2, 4].
Understanding the factors that may influence these geographic patterns is crucial to help identify the disease determinants. Within the UK the geographic patterns observed have been consistently associated with patterns of socioeconomic deprivation, at all geographic levels [8–16]. At the national level, the map of Perthes’ disease distribution is an almost identical pattern to that seen in many diseases of deprivation, such as cardiovascular disease and all-cause mortality [17]. At the local level, SOA deprivation quintiles closely correlate with the Perthes’ disease incidence rates [9] (Table 58.1). Individual deprivation measures (i.e. recording parental occupation) add support to the association with deprivation [10, 15], and this robust association has also been demonstrated to be apparent outside the UK [18]. The strength and magnitude of the association is such that few other childhood diseases demonstrate such a striking association.
Table 58.1
Incidence of Perthes’ disease by Index Multiple Deprivation 2007 Quintile at SOA level, Merseyside, UK 1996–2009
Quintile | Incidence | 95 % CI |
|---|---|---|
5 (most deprived) | 11.5 | 9.0–14.5 |
4 | 11.7 | 9.1–14.9 |
3 | 7.0 | 4.9–9.8 |
2 | 6.3 | 4.3–8.9 |
1 (least deprived) | 3.8 | 2.3–6.0 |
However, despite the strength of the deprivation association, it does not appear to offer an explanation to the international distribution of Perthes’ disease. Affluent parts of Northern Europe are shown to have high rates of disease compared to less affluent areas such as India. Similarly, while race is clearly important, it does not explain the entire observed difference in disease rates. The independent association of latitude is interesting, particularly as these latitude associations have been demonstrated in other diseases, such as multiple sclerosis and heart disease, which are believed to be a consequence of sunlight exposure and vitamin D synthesis [19, 20]. This may offer future avenues of exploration.
58.2.3 Time
Few studies have measured the influence of time on disease incidence. A study from Dortmund, Germany, between 1924 and 1960 demonstrated fluctuations in case numbers following periods of economic recession [19]. However, uncertainties in case ascertainment and the denominator population raised doubts to the validity of this observation.
More recent studies using several databases from the UK have investigated Perthes’ disease incidence over a period of up to 35 years [8, 9, 11, 14]. All of these studies have demonstrated a significant gradual decline in the incidence of Perthes’ disease. These declines were most rapid in the most deprived regions. The size and trend of this decline was similar to general markers of population well-being over this period, such as infant mortality and sudden infant death syndrome [21, 22].
58.3 Analytical Epidemiology
The descriptive studies have indicated that the key etiological determinant is intertwined with deprivation and appears to act at a single vulnerable period. The purpose of analytical epidemiology is to develop the hypotheses generated by descriptive studies and anecdotal observation. The objective is to refine the timing of the critical period, to identify additional associations that may enhance understanding of the determinant, and to test specific hypotheses of potential determinants and their mechanism of action.
58.3.1 Growth and Stature
Studies have consistently demonstrated anthropometric abnormalities among affected children. The most detailed of such study was a large cross-sectional study of 232 children from three centers in the UK [23]. This demonstrated a subtle global growth disturbance in affected individuals with a normal head size, yet increasing growth restriction more distally along limbs – a pattern of dysmorphism described as “rostral sparing” (Fig.58.3). A later study sought to determine if the growth restriction was a true disease phenomena or a function of deprivation [24]. This was achieved by comparing anthropometric markers with siblings of affected children, therefore matching for urban deprivation and family tendencies. This study of 38 cases and 49 sibling controls demonstrated a clear growth restriction most notable in the feet of affected children.
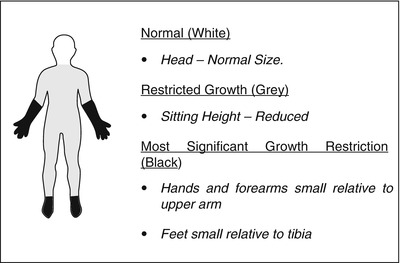

Fig. 58.3
Pattern of abnormal growth in Perthes’ disease (Reproduced with permission from Perry et al. [25])
The dysmorphic growth seen was similar to that of intrauterine growth restriction, with head size preserved at the expense of other body parameters. This suggested that there may be an abnormality evident with shape and size from birth and potentially arising in the prenatal period.
58.3.2 Birth Measures
Birth weight is difficult to study as it is complicated by the number of confounding factors such as gestational age, deprivation, and parental smoking. Numerous studies have sought to quantify this association, but no consistent association has been identified [2, 26, 27]. The most comprehensive study to investigate this used the Swedish Inpatient Register which concluded that, after adjusting for confounding, birth weights less than 1,500 g had an association with Perthes’ disease (OR 3.5 95 % CI 1.1–10.8) [28]. The width of the confidence intervals reflects the paucity of cases within this group (9 of 731 cases). It is possible that unmeasured confounders may be apparent, such as steroids used for neonatal chronic lung disease, which may explain this association. It does therefore seem apparent that no major association with birth weight exists.
58.3.3 Congenital Malformations
A possible indication of prenatal exposures may be the presence of congenital malformations. A 1971 study identified an association between with both inguinal hernias and genitourinary malformations, with the frequency of inguinal hernia being eight times the expected number among individuals with Perthes’ disease [29]. However, the patient group was selected from a specialized clinic in a tertiary center, and therefore selection bias may have influenced the validity of this finding. Subsequent studies failed to replicate these associations [5, 30] until recently, when large population-based case-control studies confirmed these associations. These studies used the Medical Birth Registry of Norway and the UK General Practice Research Database (GPRD) and confirmed a triad of genitourinary associations including undescended testis, inguinal hernias, and hypospadias, though the magnitude of these was less than previously suggested [2, 31].
These findings are important because they suggest a prenatal component to the disease etiology. The triad of associations identified is also interesting because they are often seen in combination, thought to be caused by altered fetal androgen metabolism [32, 33], which may be important in Perthes’ disease, and could explain the strong male disease preponderance of the disease.
58.3.4 Smoking Hypothesis
The association with socioeconomic deprivation, epidemiological similarities with cardiovascular disease, and possible prenatal insult has led to the suggestion that maternal tobacco smoke exposure may be implicated in the disease. Measuring the effects of tobacco smoke exposure is fraught with difficulties, owing to recall bias, parental guilt, and the influence of confounding factors (particularly socioeconomic status). Several studies have investigated this relationship, the most comprehensive of these being a case-control study from the Swedish Inpatient Register [28] and a large case-control study from India [18]. The Swedish study was able to reduce recall bias by using tobacco smoke exposure data collected prior to disease onset, demonstrating a dose-response relationship with a greater risk of Perthes’ disease corresponding to a greater degree of tobacco smoke exposure (1–9 cigarettes, OR 1.4 (95 % CI 1.1–1.7); >10 cigarettes, OR 2.0 (95 % CI 1.6–2.6)). The authors considered socioeconomic status as a confounder, although it is unclear how this was measured. The study from India similarly showed an association with tobacco smoke exposure (OR 2.1 (95 % CI 1.3–3.3)), recording current exposure to indoor tobacco smoke as a proxy measure of “lifetime exposure” and adjusting for social class. A tobacco association therefore appears evident, though the effects of bias cannot be completely eliminated, and the effect size is less than that of socioeconomic deprivation suggesting that a different factor confounded by the smoking relationship may be the responsible determinant.
58.3.5 Nutrient Hypothesis
Manganese deficiency has a known association with a disproportionate growth abnormality found in chickens and epiphyseal dysplasia in rats. A case–control study within Liverpool demonstrated the existence of this association among children with Perthes’ disease [34], but a later second smaller study failed to replicate this finding [35]. This association therefore remains uncertain.
58.3.6 Coagulopathic Hypothesis
Thrombotic tendencies , such as sickle cell disease, are known to precipitate avascular necrosis of the hip. This has led to widespread belief that Perthes’ disease is the result of a primary coagulopathic process. This appears plausible as the pathology of Perthes’ disease is avascular necrosis; however, it is difficult to resolve how such a process would solely affect the hip and typically be unilateral.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree