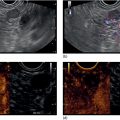
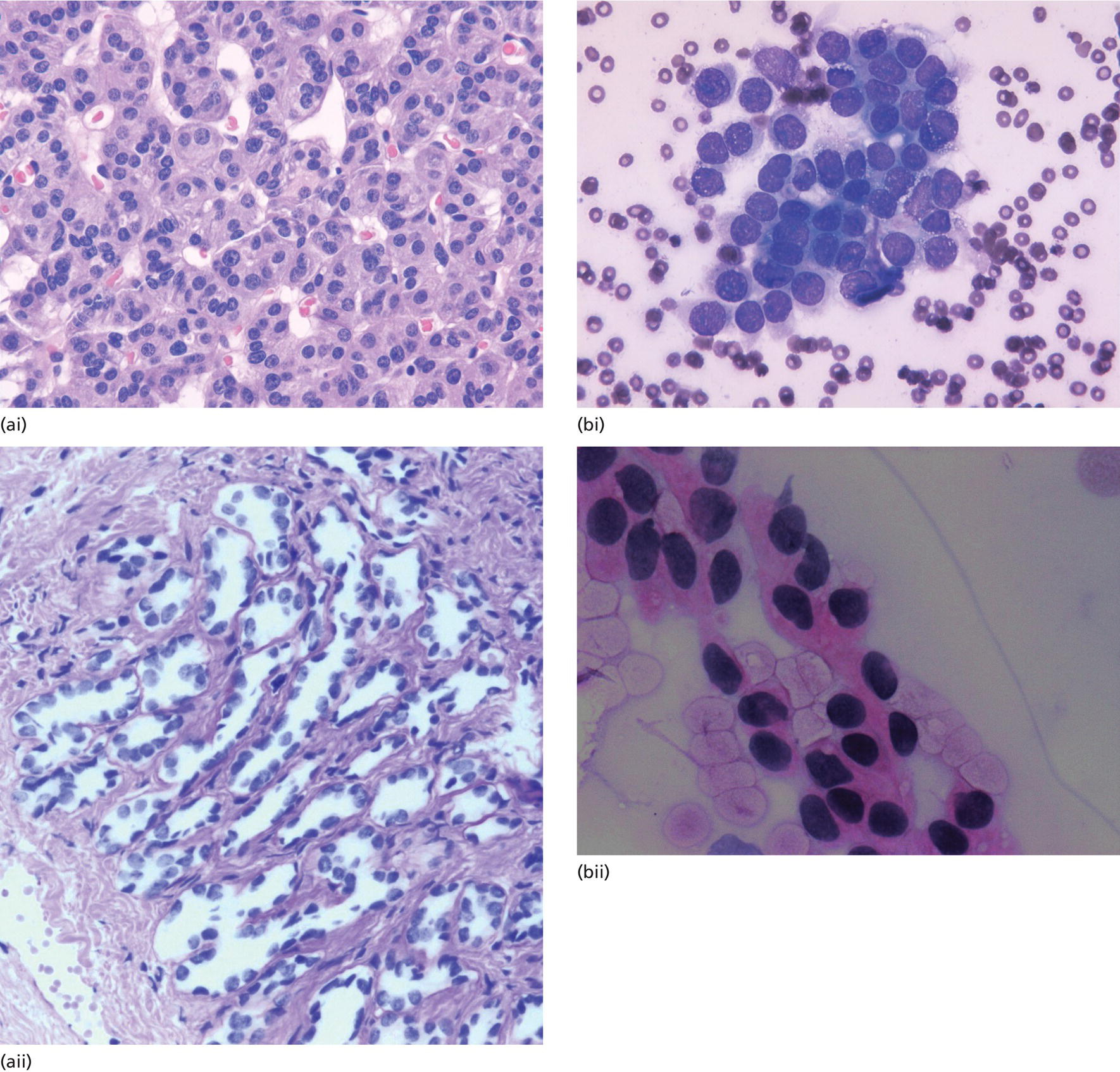
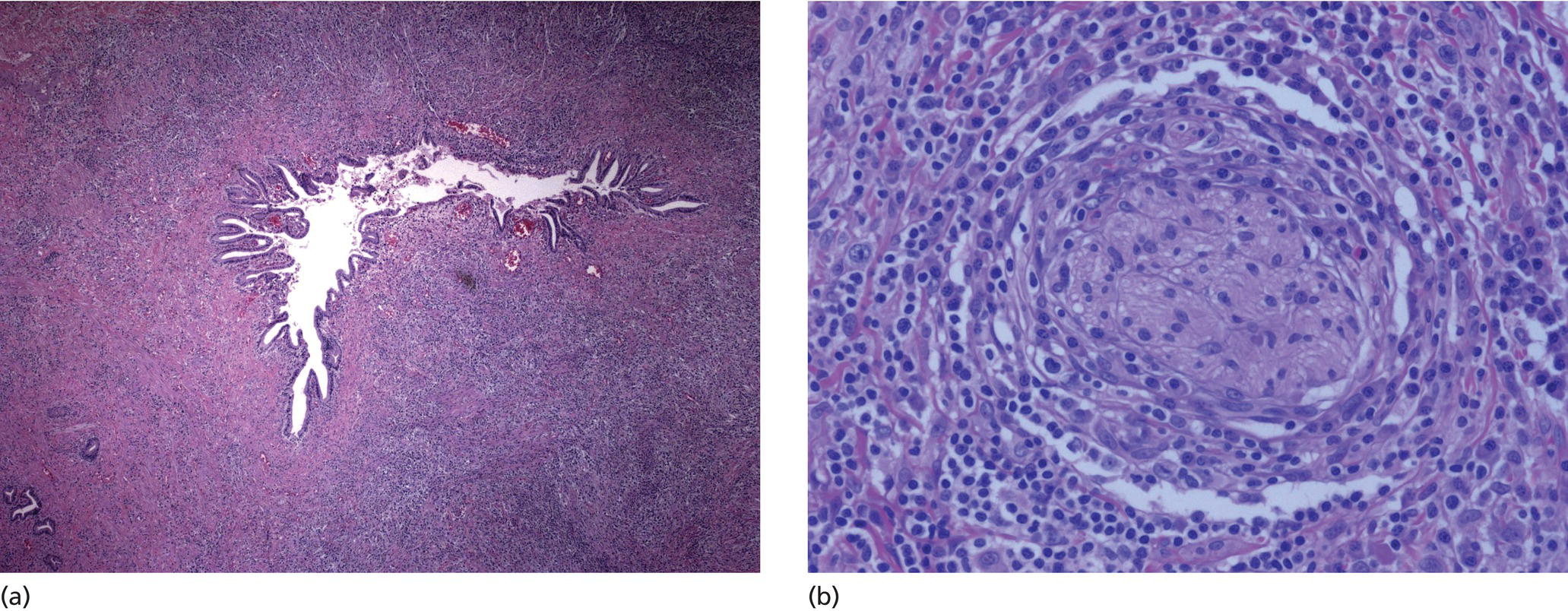
Cynthia Behling Pacific Rim Pathology Group, Sharp Memorial Hospital, San Diego, CA, USA Endoscopic ultrasound (EUS) is an ideal method for obtaining tissue from submucosal lesions in the gastrointestinal tract or from organs near the gastrointestinal tract. A pathologic interpretation of the tissue biopsy in addition to the EUS characteristics helps to accurately determine the nature of the lesion. The pathologist represents an important member of the patient care team, since microscopic evaluation is key in determining the nature of the sampled lesion. This chapter illustrates some common lesions as well as technical issues. The chapter is divided into three sections which relate pathology and EUS: the technical quality of the material; the quality of interpretation; and the integration of pathologic and clinical information. The goal of the pathologist is to contribute to a successful EUS service by providing accurate and precise diagnostic information. Although EUS‐guided sampling is a relatively newer way of obtaining tissue, the means of preparing the cells or tissue, obtained from endoscopic ultrasound‐guided fine needle aspiration (EUS‐FNA), referred to in general as a “biopsy,” are well‐developed and standardized procedures in most cytology/anatomic pathology laboratories. In this chapter, the term “biopsy” refers to any tissue sample obtained via EUS guidance. A “biopsy” could be an FNA, representing cells obtained through a small needle and dispersed onto slides, or a core, representing an intact piece of tissue. The advantages and disadvantages of each type of biopsy are discussed later. There are a number of techniques and strategies which can optimize the technical quality of EUS biopsy material. Before developing an EUS service, it is important to involve a pathologist or cytotechnologist in planning for specimen handling. Selection of biopsy type and other procedures depends on the personnel and laboratory resources available. Use of a liquid‐based cytologic preparation such as ThinPrep™ or SurePath™, direct smears, core biopsy, or some combination of these methods depends on the lesion, pathologist and endoscopist preference, staffing, location of the endoscopy suite in relation to the laboratory, and the relative sensitivity, specificity, and diagnostic accuracy of the various technical choices. Personnel issues are key in planning for the actual EUS service. For example, at some institutions, the laboratory is physically close to the endoscopy suite and a laboratory technologist travels to the site to prepare aspirate smears, while in other practices the gastrointestinal endoscopy staff are trained to make slides. If laboratory personnel will help prepare specimens, the scheduling of procedures should be done in consideration of the laboratory schedule and any ancillary tests such as flow cytometry, which might require special processing. There are two ways of obtaining biopsy material from a lesion. One is to cut out a small piece of the lesion with a cutting needle and the other to aspirate individual cells and small fragments of tissue. Core biopsies provide tissue architecture, that is, the relationship of the lesional cells and their surrounding stroma are maintained. In core biopsies, vascular and duct structures usually remain intact (Figure 29.1a). In an aspirate sample, the cells are dispersed and evaluated primarily for their individual characteristics, although some microarchitecture remains (Figure 29.1b). Hematoxylin and eosin (H&E)‐stained 3–5‐μm sections are cut from tissue obtained with a core biopsy, and are typically fixed in formalin, processed, and embedded in a paraffin block. The procedure is essentially identical to the way in which mucosal biopsies from the gastrointestinal tract are processed. These are the familiar blue and pink, hematoxylin and eosin slides with which all pathologists and gastroenterologists are familiar. For FNA, biopsies are dropped onto a glass slide and then dispersed as a monolayer by drawing a second slide over the fluid drop. Figure 29.1 (a) Core biopsies demonstrate intact tissue architecture and structures such as ducts, vessels, and stromal relationships. (ai) Pancreatic neuroendocrine tumor showing fine vascular structures and rosette formation. (aii) Diastase digested PAS stain of a serous cystadenoma of the pancreas showing small cysts in a fibrous stroma. (b) The dispersed preparation of cells from a fine needle aspirate allow evaluation of individual nuclear and cytoplasmic features as well as microarchitecture. (bi) Pancreatic neuroendocrine tumor cells have finely dispersed chromatin, small nucleoli, and little cytoplasm. (bii) Fine needle aspirates from serous tumors usually contain few cells with oval nuclei arranged in small glandular structures. An aspirate biopsy is usually obtained with a “fine” needle, which is 22 gauge or smaller. In this type of biopsy, the material flows or is actively aspirated into the needle and then dispersed onto a slide or placed directly into a preservative or fixative for processing. Smears are prepared in a manner similar to that used in making blood smears. A small drop of the aspirate is placed on one end of the slides and then carefully pulled along the slide to make a dispersed layer of individual and small groups of cells. The features of the cells appear somewhat different in smears than in tissue sections and also depend on the type of smear preparation and staining. In many pathology laboratories, aspirate smears are prepared as both air‐dried and alcohol‐fixed smears. Figure 29.2 Diagnostic features of autoimmune pancreatitis rely on the demonstration of duct centric inflammation (a), and a dense plasmacytic infiltrate, often surrounding nerves (b). Different criteria are used in interpreting tissue sections and cytology smears. In the tissue sections evaluable features include formation of structures such as glands, invasion of stroma or nerves, inflammatory changes in duct structures, and many others (Figure 29.2). Preservation of the architecture allows observation of features such as the shape of glands, quality of the surrounding stroma, and the presence of vasculitis. Aspirate smears rely on visualization of nuclear and chromatinic detail, nuclear membrane contour, and cytoplasmic quality (Figure 29.3), emphasizing features of individual cells. Endoscopic ultrasound‐guided sampling lends itself to either aspiration or acquisition of a tissue core (with few exceptions). In some procedures both types of biopsy are obtained, in order to capitalize on the advantages of both preparations. However, for some diseases such as autoimmune pancreatitis or lymphoma, diagnostic criteria may be dependent on features of intact tissue. Core biopsy tissue remaining in the paraffin block after the initial sections are taken can be used for special stains. Special stains are not limited to tissue biopsies; however, since a portion of the aspirated material from each needle pass can be aliquoted into cell culture media such as RPMI or into a preservative and the sample centrifuged and the pellet processed, embedded, and sectioned. This cell block made from aspirated material can be similarly used for ancillary studies (Figure 29.4). While examination of tissue sections is the core practice of pathologists, not all pathologists practice cytopathology and, in general, more pathologists are comfortable interpreting tissue sections. For this reason and others, some believe that a tissue core is a better choice. The issue of core versus aspirate can be debated, although the two methods are complementary. Various strategies of “dual sampling” or sequential sampling, that is, using FNA when the core is macroscopically inadequate, have been examined. For some situations, such as autoimmune pancreatitis, a core biopsy may provide better diagnostic material. The tissue architecture of a core biopsy may be useful for traditional diagnostic criteria for lymphomas, and can help distinguish invasive and in situ malignancies or very well differentiated malignancies such as some pancreatic carcinomas. In addition, core biopsy may require fewer needle passes for diagnostic material. Endoscopic ultrasound‐guided core biopsies are a recent technical development and have not been studied as completely as EUS‐FNA, so the true diagnostic accuracy remains to be determined. A potential advantage of core biopsy is that tissue remains in the paraffin block and is available for special stains or other studies as needed. Core biopsy may lead to a more confident benign diagnosis when used in combination with FNA for mediastinal lesions but may be less sensitive in pancreas.
29
How to Interpret EUS‐FNA Cytology
Introduction
Technical quality of EUS biopsy material
Personnel
Biopsy type: aspirate or core
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree