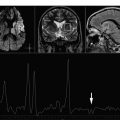
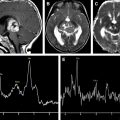
Hypoxic ischemic injuries are a very common clinical situation in the pediatric population. This article focuses on the metabolic signature of hypoxic ischemic injuries and metabolic indicators of prognosis.
Key points
- •
Magnetic resonance spectroscopy (MRS) is perhaps more sensitive to injury and more indicative of the severity of injury in the first 24 hours after a hypoxic-ischemic episode, when conventional magnetic resonance imaging and diffusion-weighted imaging may yield false-negative findings or lead to significant underestimation of the extent of injury.
- •
Neonates with asphyxia have statistically significant correlations between proton MRS results and both neurologic and cognitive status by 12 months of age.
- •
The optimal regions for determining the abnormalities associated with hypoxic injury include the occipital cortex, the basal ganglia, and watershed zones.
- •
Lactate is an early observation within the first 12 to 24 hours after insult, and is the best prognostic indicator in the early stage.
- •
In the late stage, N -acetyl-aspartate is the preferred prognostic indicator.
- •
Proton MRS should be performed as early as possible, preferably in the first week of life, so that abnormalities caused by hypoxia can be identified.
Introduction
Several conditions may result in hypoxic encephalopathy (HE):
- •
Low Apgar score at birth
- •
Neonatal asphyxia/hypoxemia
- •
Severe asthma
- •
Drowning
- •
Congestive heart failure
- •
Extensive myocardial infarction
- •
Congenital cardiopathy
- •
Bronchoaspiration
- •
Head injury
- •
Respiratory failure
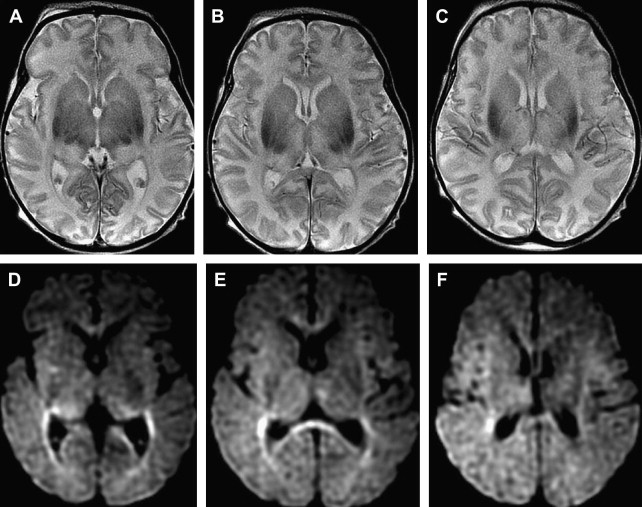
Although computed tomography and magnetic resonance imaging (MRI) are useful for assessing hypoxic injury, changes in images often do not occur immediately. In addition, the lack of myelination in the neonatal brain can obscure white matter hypoxic ischemic changes on T2-weighted images ( Fig. 1 ).
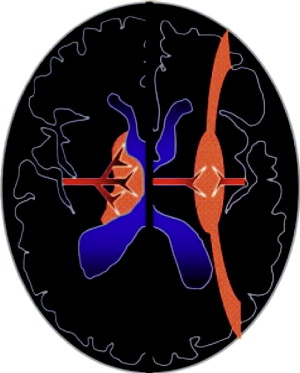
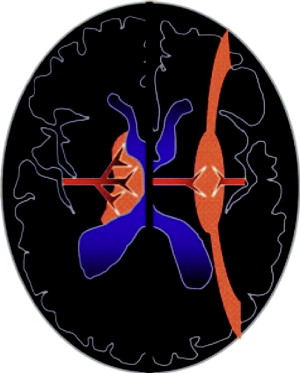
Distinct patterns of brain injury are observed and result from various combinations of 3 primary factors: the level of brain maturation at the time of the insult and the severity and duration of the hypoperfusion event. The vascular supply to the brain changes with brain maturation. In the immature brain, ventriculopetal penetrating arteries extend inward from the surface of the brain to supply the periventricular regions; hence, periventricular leukomalacia is the most common pathologic finding in hypoperfusion injury. With maturation of the brain (36 weeks’ gestation), vessels extend into the brain from the lateral ventricles, and the intervascular border zone moves peripherally to a parasagittal location ( Fig. 2 ).
A hypoxic-anoxic event lasting more than 10 minutes is required to induce parenchymal changes, and the extent of injury increases with prolonged duration of the insult.
Introduction
Several conditions may result in hypoxic encephalopathy (HE):
- •
Low Apgar score at birth
- •
Neonatal asphyxia/hypoxemia
- •
Severe asthma
- •
Drowning
- •
Congestive heart failure
- •
Extensive myocardial infarction
- •
Congenital cardiopathy
- •
Bronchoaspiration
- •
Head injury
- •
Respiratory failure
Although computed tomography and magnetic resonance imaging (MRI) are useful for assessing hypoxic injury, changes in images often do not occur immediately. In addition, the lack of myelination in the neonatal brain can obscure white matter hypoxic ischemic changes on T2-weighted images ( Fig. 1 ).
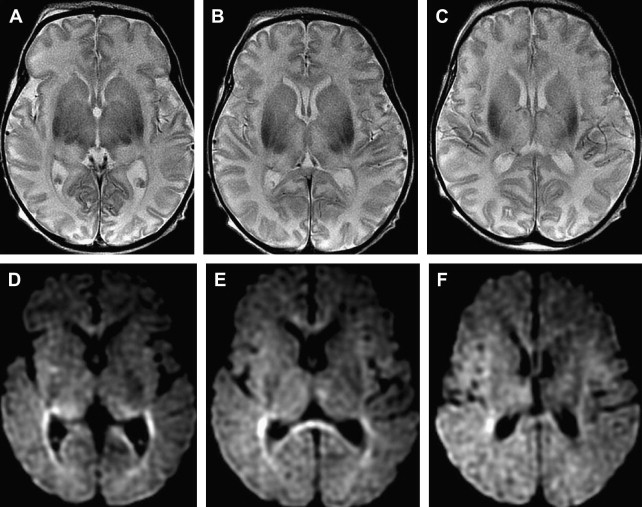
Distinct patterns of brain injury are observed and result from various combinations of 3 primary factors: the level of brain maturation at the time of the insult and the severity and duration of the hypoperfusion event. The vascular supply to the brain changes with brain maturation. In the immature brain, ventriculopetal penetrating arteries extend inward from the surface of the brain to supply the periventricular regions; hence, periventricular leukomalacia is the most common pathologic finding in hypoperfusion injury. With maturation of the brain (36 weeks’ gestation), vessels extend into the brain from the lateral ventricles, and the intervascular border zone moves peripherally to a parasagittal location ( Fig. 2 ).
A hypoxic-anoxic event lasting more than 10 minutes is required to induce parenchymal changes, and the extent of injury increases with prolonged duration of the insult.
Proton magnetic resonance spectroscopy: clinical applications
Proton magnetic resonance spectroscopy (MRS) has proven very useful in the assessment of patients with HE.
MRS and diffusion-weighted imaging (DWI) are the most sensitive imaging modalities for detecting hypoxic ischemic encephalopathy (HIE) in the acute period.
MRS is perhaps more sensitive to injury and more indicative of the severity of injury in the first 24 hours after a hypoxic-ischemic episode, when conventional MRI and DWI may yield false-negative findings or lead to significant underestimation of the extent of injury.
Proton MRS may show abnormalities earlier than conventional MRI, documenting the severity of the encephalopathy and predicting the neuropsychological and motor development of patients. Neonates with asphyxia show statistically significant correlations between proton MRS results and both neurologic and cognitive status by 12 months of age. Nevertheless, it is important to correlate the MRS findings with MRI findings to interpret the spectra properly.
Clinical progression can be followed by serial spectroscopy.
Voxel positioning
Because HE is a global event, single voxel spectroscopy can be used to assess it. The optimal regions for determining the abnormalities associated with hypoxic injury include the occipital cortex ( Fig. 3 ), basal ganglia ( Fig. 4 A), and watershed or border zones (see Fig. 4 B).