Neurodegenerative dementias are characterized by elevated myoinositol and decreased N -acetylaspartate (NAA) levels. The increase in myoinositol seems to precede decreasing NAA levels in Alzheimer’s diseases. NAA/ myo -inositol ratio in the posterior cingulate gyri decreases with increasing burden of Alzheimer’s disease pathologic conditions. Proton magnetic resonance spectroscopy ( 1 H MRS) is sensitive to the pathophysiologic processes associated with the risk of dementia in patients with mild cognitive impairment. Although significant progress has been made in improving the acquisition and analysis techniques in 1 H MRS, translation of these technical developments to clinical practice have not been effective because of the lack of standardization for multisite applications and normative data and an insufficient understanding of the pathologic basis of 1 H MRS metabolite changes.
Key points
- •
Neurodegenerative dementia is characterized by elevated myoinositol and decreased N -acetylaspartate (NAA) levels.
- •
The increase in myoinositol seems to precede decreasing NAA levels in Alzheimer’s disease.
- •
NAA/ myo -inositol ratio in the posterior cingulate gyri decreases with increasing burden of Alzheimer’s disease pathologic conditions.
- •
Proton magnetic resonance spectroscopy ( 1 H MRS) is sensitive to the pathophysiologic processes associated with the risk of dementia in patients with mild cognitive impairment.
- •
Although significant progress has been made in improving the acquisition and analysis techniques in 1 H MRS, translation of these technical developments to clinical practice have not been effective because of the lack of standardization for multisite applications and normative data and an insufficient understanding of the pathologic basis of 1 H MRS metabolite changes.
Introduction
Aging is the primary risk factor for dementia. With increasing life expectancy and the aging populations around the world, dementia is becoming a significant public health problem of the century. The most common pathologic condition underlying dementia in older adults is Alzheimer’s disease (AD). Cerebrovascular disease and Lewy body disease pathologic conditions are other common causes of dementia in the elderly, and in many instances AD is also present in patients with dementia with Lewy bodies (DLB) and vascular dementia. A relatively less common type of dementia is frontotemporal lobar degeneration, which tends to affect younger individuals compared with other dementia pathologies. The focus of this article is potential role of proton magnetic resonance spectroscopy ( 1 H MRS) in these common dementias.
Introduction
Aging is the primary risk factor for dementia. With increasing life expectancy and the aging populations around the world, dementia is becoming a significant public health problem of the century. The most common pathologic condition underlying dementia in older adults is Alzheimer’s disease (AD). Cerebrovascular disease and Lewy body disease pathologic conditions are other common causes of dementia in the elderly, and in many instances AD is also present in patients with dementia with Lewy bodies (DLB) and vascular dementia. A relatively less common type of dementia is frontotemporal lobar degeneration, which tends to affect younger individuals compared with other dementia pathologies. The focus of this article is potential role of proton magnetic resonance spectroscopy ( 1 H MRS) in these common dementias.
MRS in AD
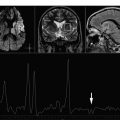
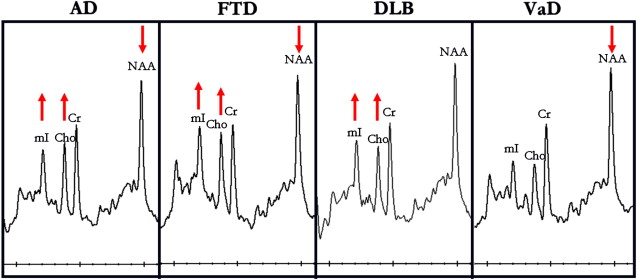
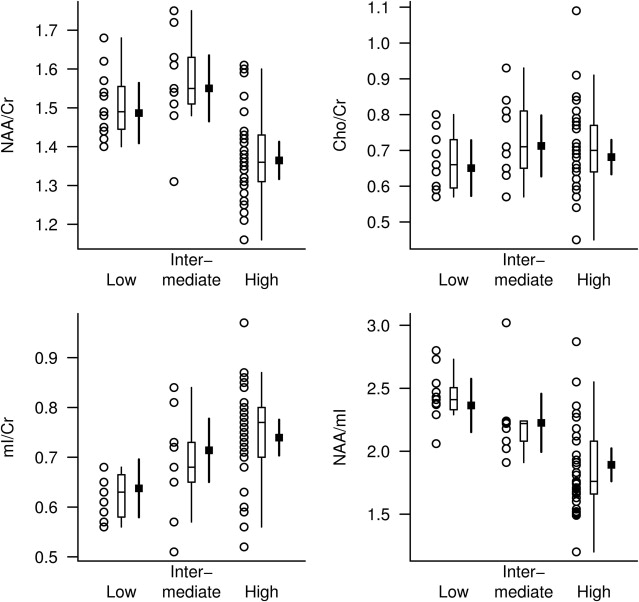
Initial MRS studies in AD were limited to phosphorous magnetic resonance spectroscopy ( 31 P MRS) revealing alterations in membrane phospholopid metabolism. In 1992, Klunk and colleagues demonstrated the decrease in the neuronal metabolite N -acetylaspartate (NAA) on 1 H MRS using perchloric acid extracts from brains from persons with AD. Soon after, an in vivo MRS study revealed elevated myo- inositol/creatine (mI/Cr) levels in patients with AD along with decreased NAA/Cr. Further investigations in patients with AD confirmed this finding ( Fig. 1 ). Many of these early studies also revealed that the increase in mI/Cr and decrease in NAA/Cr in AD were not associated with a change in Cr using absolute quantification methods. For this reason. Cr is commonly used as an internal reference in MRS studies of AD to account for individual and acquisition related variability. There have been conflicting reports on choline (Cho) levels in AD. Some studies found elevated Cho or Cho/Cr levels, whereas others found no changes in Cho or Cho/Cr levels in AD. Decreased glutamate plus glutamine levels have been found in several studies of AD.
Regional alterations of 1 H MRS metabolites in patients with AD seem to be widespread, involving the parietal, medial and lateral temporal, and frontal lobes. Furthermore, decreases in NAA or NAA/Cr correlate with dementia severity, cognitive function, and behavioral and psychiatric symptoms, indicating that NAA is a marker for AD severity on various clinical features.
Most of the 1 H MRS studies in AD have used single-voxel 1 H MRS. Choice of the single-voxel 1 H MRS region for detecting and monitoring metabolite levels in AD depends on both the pathophysiology of AD and the technical considerations. The neurofibrillary pathologic conditions of AD and associated neuronal loss involve medial temporal lobes earlier and more severely than other regions of the brain. Although 1 H MRS from the medial temporal lobe or hippocampus yields spectra of reasonable quality using long echo times (TE = 130–272 milliseconds), it may be more difficult to achieve MR spectra of consistent quality from the hippocampus using short echo times required for quantification of the mI peak (TE <35 milliseconds). At 4 T using adiabatic selective refocusing for localization and TE of 46 milliseconds, it was possible to measure decreased glutamate levels from the hippocampus in AD. Another region that is commonly investigated in single voxel MRS studies of AD is the posterior cingulate gyrus voxel. Posterior cingulate gyrus is severely involved with the β-amyloid (Aβ) pathologic condition of AD and 18 F fluorodeoxyglucose positron emission tomography (PET) studies suggest that the synaptic activity is reduced in this region very early in the disease process such as in cognitively normal carriers of the APOE ε4 allele who are at a higher risk for AD than noncarriers. Recently, it was demonstrated that posterior cingulate gyrus is the hub for the resting state connectivity networks on task-free functional MRI, which are affected by AD early in the disease course. It is possible to consistently obtain acceptable-quality short-echo time MR spectra from the posterior cingulate gyrus voxel for quantification of mI levels, which is critical for longitudinal evaluations and multisite studies.
Human tissue studies in transgenic mouse models of AD have provided some insight into the pathologic underpinnings of decreased NAA and glutamate levels and increased mI levels, which closely resemble the metabolic abnormalities observed in patients with AD. For example, lower NAA and glutamate levels were associated with Aβ plaque load in mice with PS2APP mutation. Magic angle spinning 1 H MRS in superior temporal cortex tissue from patients with AD showed a correlation between NAA concentration and neuronal density. Recently, an in vivo 13 carbon ( 13 C)-MRS and 1 H MRS study suggested a link between increased glial or microglial activation and mI elevation in AD.
Our investigation of pathologic correlates of MRS metabolite changes in 54 cases with varying degrees of AD pathologic conditions demonstrated that both NAA/Cr and mI/Cr levels measured antemortem are associated with the pathologic classification of AD severity. Whereas mI/Cr ratio was elevated at earlier stages of pathologic involvement (ie, intermediate likelihood AD), NAA/Cr levels were decreased only at the late stage of pathologic involvement (ie, high likelihood AD). A combined ratio of the 2 metabolites as the NAA/mI, however, revealed the strongest association with pathologic severity, suggesting that both NAA and mI provide complementary information on AD pathologic conditions ( Fig. 2 ).
Longitudinal MRS studies in patients with AD demonstrate gradually decreasing NAA, NAA/Cr, and NAA/mI levels compared with controls. The changes in NAA and NAA/Cr also correlate with the cognitive decline. Although the data are limited, no study has yet reported a longitudinal increase in mI or mI/Cr levels in patients with AD. The reasons may be 2-fold: (1) lower reliability of mI quantification compared with NAA and (2) stage of the AD pathologic process. If the elevation of mI is an early event in the pathologic progression of AD, it is possible that a plateau is reached in the mI elevation toward the later stages of the pathologic process, which remains to be investigated.
The applications of MRS as a biomarker for treatment response in clinical trials have been limited to single-site studies. The change in NAA/Cr correlated with the change on the Alzheimer’s Disease Assessment Scale, cognitive part scores during a cholinesterase inhibitor treatment trial. Short-term or temporary increases in NAA/Cr and Glu/Cr was observed in cholinesterase inhibitor–treated AD patients versus placebo and one trial found no effect on NAA/Cr ratio but found a decrease in Cho/Cr and mI/Cr ratios in the hippocampus, albeit in the absence of cognitive response. Overall, MRS seems to be a feasible biomarker in single-site clinical trials in AD. Efforts to extend these applications to multisite clinical trials are under way. Larger sample sizes may be needed for MRS compared with structural MRI measurements (eg, hippocampal volumes) to detect a similar effect size. However, effect sizes of a single treatment may differ among imaging markers of different pathophysiologic processes. For example, a treatment that improves neuronal function may dramatically improve NAA levels but not significantly alter atrophy rates. Therefore, effects of the intervention should be considered when comparing imaging markers of different biologic sensitivity on sample size and power.
MRS in DLB
The presence of Lewy bodies in substantia nigra is the pathologic feature of Parkinson’s disease. Although cortical Lewy bodies can occasionally be detected in Parkinson’s disease, cortical Lewy bodies presenting with dementia is recognized as DLB, a distinct neurodegenerative disease with established clinical and pathologic criteria. DLB is frequently accompanied by AD in patients with dementia. Lewy body pathologic condition by itself is less common than the mixed (AD and DLB) type. In our 1 H MRS series, patients clinically diagnosed as probable DLB have normal NAA/Cr levels, whereas patients with AD, vascular dementia, and frontotemporal dementia have lower NAA/Cr levels than normal, in the posterior cingulate gyri. Normal NAA/Cr levels in the posterior cingulate gyri in patients with DLB suggest integrity of neurons in this region, which is in keeping with the preserved neuronal numbers found in the neocortex of DLB patients at autopsy. Normal neocortical NAA or NAA/Cr levels may be useful in distinguishing patients with DLB from those with other dementia syndromes. Reduced NAA/Cr has been detected in the hippocampi of patients with DLB ; however, many patients with DLB may have hippocampal atrophy because of the presence of concomitant neurofibrillary tangle pathologic conditions of AD, making it hard to determine whether the low NAA/Cr levels in DLB is associated with the coexistent AD pathologic conditions. One study found reduced white matter NAA/Cr in patients with DLB compared with the control group, suggesting white matter involvement in DLB. The white matter involvement in DLB was later confirmed with diffusion tensor imaging studies.
We found elevated Cho/Cr in the posterior cingulate gyri of patients with DLB (see Fig. 1 ). Elevation of Cho in DLB and AD may be the consequence of increased membrane turnover caused by dying back of the neuropil. Another explanation, however, is down-regulation of choline acetyltransferase activity, which may be responsible for this change in both AD and DLB. Activity of choline acetyltransferase, the enzyme responsible for acetylcholine synthesis from free Cho, is reduced earlier and more severely in the disease course of DLB than in that of AD. Furthermore, patients with DLB who were treated with cholinesterase inhibitors have shown substantial cognitive improvement, revealing the functional significance of acetylcholine deficiency in DLB. The finding that Cho/Cr levels decrease with cholinergic agonist treatment in AD raises the possibility that Cho/Cr levels may be a biomarker of therapeutic efficacy in both AD and DLB treatment trials.
MRS in vascular dementia
Cerebrovascular disease is another common pathologic condition observed in patients with dementia in autopsy series. In most cases, however, vascular pathologic conditions coexist with the pathologic conditions of AD, and a pure vascular pathologic condition is relatively uncommon. Vascular lesions are more common in patients with dementia than in the normal elderly. In a patient with the clinical diagnosis of AD and cerebrovascular disease, the challenge is to identify how much, if any, of the 2 pathologic conditions are contributing to dementia, so that appropriate therapies can be planned. MRS studies indicate that NAA and NAA/Cr levels are reduced in patients with vascular dementia. White matter NAA/Cr is lower in patients with vascular dementia than in patients with AD, reflecting the white matter ischemic damage in vascular dementia with respect to the cortical degenerative pathologic conditions in AD. NAA levels are further decreased in patients with stroke who have cognitive impairment compared with those who are cognitively normal even in regions remote from the infarction, suggesting NAA/Cr is a marker for neuronal dysfunction that may extend beyond the region of infarct. Cortical mI/Cr levels on the other hand are normal in patients with vascular dementia (see Fig. 1 ). Because mI/Cr is elevated in patients with AD, mI/Cr may help identify the presence of AD in a demented patient with cerebrovascular disease. Studies that include histopathologic confirmation are necessary to clarify the role of mI/Cr in differential diagnosis of vascular dementia, mixed dementia (vascular dementia and AD), and AD.
MRS in frontotemporal lobar degeneration
MRS metabolite changes in frontotemporal dementia are similar to the changes encountered in AD: lower NAA/Cr and higher mI/Cr than normal (see Fig. 1 ). NAA/Cr is lower and mI/Cr is higher in the frontal cortex of patients with frontotemporal dementia than in patients with early AD, suggesting that regional 1 H MRS measurements may help differentiate neurodegenerative disorders that display regionally specific involvement. It should be noted, however, that although regional differences may be prominent during early stages of the pathologic process in neurodegenerative diseases, these differences may be lost as neurodegenerative pathologic conditions involve most of the cerebral cortex in later stages.
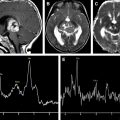
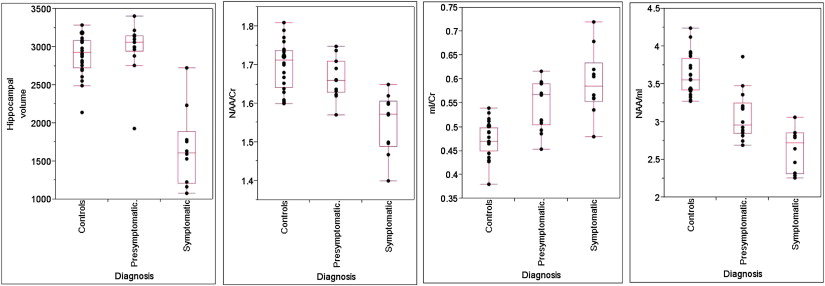
Frontotemporal dementia with parkinsonism linked to chromosome 17 is an autosomal dominant tauopathy that is linked to mutations in the gene encoding for the microtubule-associated protein tau ( MAPT ) on chromosome 17. Mutations in MAPT result in filamentous accumulation of hyperphosphorylated tau in neurons and glia leading to neurodegeneration and atrophy. Progressive accumulation of filamentous tau and subsequent neuronal death is central to the pathogenesis of many neurodegenerative diseases, including AD, and may begin years before the onset of clinical symptoms. We recently demonstrated 1 H MRS metabolite abnormalities in presymptomatic carriers of mutations in the gene encoding for MAPT on chromosome 17. The severity of 1 H MRS and MRI abnormalities was associated with the proximity to the estimated age of symptom onset. NAA/mI ratio was fully outside of the control range in presymptomatic MAPT mutation carriers who had 5 years to reach estimated age of symptom onset or who were past the estimated age of symptom onset, indicating the presence of 1 H MRS metabolite abnormalities related to neurodegeneration, years before the onset of symptoms and atrophy in MAPT mutation carriers ( Fig. 3 ).
MRS in mild cognitive impairment and other AD risk groups
There are no proven treatments for AD pathologic conditions; however, current efforts to arrest or slow disease progression generate the prospect for preventive interventions. There is considerable interest in early diagnosis by identifying individuals with cognitive difficulties who eventually progress to dementia from those who are aging with normal cognitive function. Mild cognitive impairment (MCI) was established on clinical grounds to identify symptomatic individuals who do not meet the criteria for dementia. Most people with MCI develop dementia in the future.
The progression of AD pathophysiologic processes starts decades before the clinical diagnosis of AD and the earliest cognitive impairments occur in the memory domain. The syndrome of amnestic MCI represents this prodromal phase in the progression of AD. More recently, the construct of MCI has been broadened to include individuals with impairments in nonamnestic cognitive domains such as attention/executive, language, or visuospatial processing domains. The clinical presentation of this broadened definition of MCI is heterogeneous. Both the amnestic and nonamnestic subtypes of MCI may present with involvement of a single cognitive domain or multiple cognitive domains. It is clear from several independent studies that most people with the amnestic form of MCI who progress to dementia in the future develop AD. People with nonamnestic MCI on average have more vascular comorbidity and infarctions and a higher prevalence of extrapyramidal features, mood disorders, and behavioral symptoms than do people with amnestic MCI. The cause of MCI is also heterogeneous. A variety of early stage dementia-associated pathophysiologic processes such as AD, cerebrovascular disease and Lewy body pathologic conditions have been identified in patients with MCI at autopsy. Many of these pathologies coexist in MCI and require different therapeutic strategies. Furthermore, patients with MCI do not develop dementia at similar rates. The heterogeneity of MCI warrants development of noninvasive biomarkers that can predict the rate of future progression to different dementias, for early diagnosis and treatment with potential disease-specific preventive interventions.
Early 1 H MRS studies in MCI included individuals who had impairments in memory function (ie, amnestic MCI). Most patients with amnestic MCI develop AD in the future, and many of these individuals have early AD pathologic conditions. In keeping with this, the 1 H MRS findings in amnestic MCI are similar to but milder than the findings in AD. However, there are distinct groupwise differences in MRI and 1 H MRS findings between amnestic MCI and nonamnestic MCI subtypes. Patients with amnestic MCI tend to have smaller hippocampal volumes and elevated mI/Cr ratios compared with patients with nonamnestic MCI and cognitively normal controls. On the other hand, nonamnestic MCI patients have normal hippocampal volumes and normal mI/Cr ratios, but a greater proportion of these patients have cortical infarctions compared with the amnestic MCI patients. Both hippocampal atrophy and elevated mI/Cr are sensitive markers of early AD pathologic conditions, and the severity of these abnormalities correlates with the pathologic severity of AD. For this reason, hippocampal atrophy and elevated mI/Cr most likely represent a higher frequency of early AD pathologic conditions in patients with amnestic MCI compared with nonamnestic MCI. On the contrary, normal hippocampal volumes and mI/Cr ratios in the nonamnestic MCI subtype suggest that other pathologic conditions, in addition to AD, underlie nonamnestic MCI. A higher prevalence of cortical infarctions on MRI and a history of transient ischemic attack and stroke in nonamnestic MCI patients suggest that cerebrovascular disease is one of the pathologic contributors to nonamnestic MCI.
The pathologic and clinical heterogeneities of MCI require multimodality imaging markers that are sensitive to the various dementia-related pathophysiologic processes for early diagnosis in patients with MCI. The most common dementia-related pathologic conditions observed in MCI are AD, cerebrovascular disease, and DLB. Lesions that are associated with cerebrovascular disease on MRI include infarctions and white matter hyperintensities on T2-weighted images. These cerebrovascular lesions are more common in patients with MCI compared with cognitively normal older adults. An MR marker that is highly sensitive to the pathophysiologic processes of AD (specifically, the neurofibrillary tangle pathologic condition–associated neurodegeneration early in the disease course) is hippocampal atrophy. Both cortical infarctions and hippocampal atrophy are predictors of dementia risk in MCI.
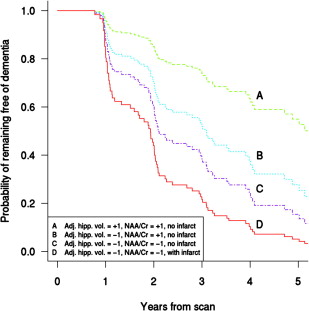
There is evidence that 1 H MRS is sensitive to the pathophysiologic processes associated with the risk of dementia in patients with MCI. Decreased NAA/Cr ratio in the posterior cingulate gyrus voxel is associated with an increased risk of dementia in patients with MCI. Furthermore, posterior cingulate gyrus voxel NAA/Cr levels decline with time in patients with MCI who progress to an AD diagnosis. 1 H MRS is complementary in predicting future progression to dementia in MCI when considered with other strong predictors of dementia risk in MCI such as hippocampal volumes and cortical infarctions. Decreased posterior cingulate gyrus NAA/Cr increases the risk of progression to dementia in patients with MCI with hippocampal atrophy, and the risk of dementia increases even further if cortical infarctions are present in a patient with MCI ( Fig. 4 ). The complementary role of multimodality imaging markers in predicting the risk of dementia in MCI is consistent with cross-sectional studies showing the added value of 1 H MRS and hippocampal volumes for discriminating cognitively impaired but nondemented individuals from cognitively normal subjects and distinguishing patients with AD from those who are cognitively normal. Furthermore, hippocampal volumes and NAA/Cr levels are independent and complementary predictors of verbal memory on neuropsychometric testing in nondemented older adults, demonstrating that verbal memory depends on both structural and metabolic integrity of the hippocampus.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree