Fig. 26.1
(a) A 125I radioactive implant using sources in suture material is stitched into an absorbable mesh to simplify placement of radioactive sources in the tumor bed after resection of the tumor. (b) The mesh with radioactive sources attached is clipped onto the post-resection tissue of the tumor bed. (c) A coronal plane reconstructed from a CT scan with the resulting dose distribution overlaid. The CT is used to identify and localize the sources within the patient. A radiation oncology treatment planning system calculates the distribution of radiation delivered in the vicinity of the implant
Temporary Brachytherapy
Temporary brachytherapy places sources in the tumor or region of interest for a limited duration. Afterloading is a method of decoupling the deployment of the radioactive sources from the procedure that provides access to the region of interest. Needles, catheters, or applicators may be placed, either as part of an open procedure or by less invasive means using image guidance. The radiation sources are delivered into the applicator later in the procedure or as part of a separate radiation therapy procedure.
The applicators can be visualized on x-ray or CT as shown in Fig. 26.2, and MR-compatible applicators are becoming more common. With the ability to image the applicator and surrounding tissue comes the ability to plan the source deployment to achieve optimal radiation dose to the target while balancing the radiation dose to nearby normal or radiation-sensitive structures, which would correspond to image-based brachytherapy planning. The short range of the radiation, which makes it possible to achieve highly conformal dose distributions, limits the ability of the treatment planning process to overcome a suboptimal applicator placement. This has led to the use of simple x-ray imaging during or at the end of the applicator placement to ensure adequate applicator placement. The assessment is usually visual, with little quantitative component. It is usually viewed as being sufficient for the rigid applicators typically used in intracavitary brachytherapy, such as cylinders or tandem and ovoids (Fig. 26.3).
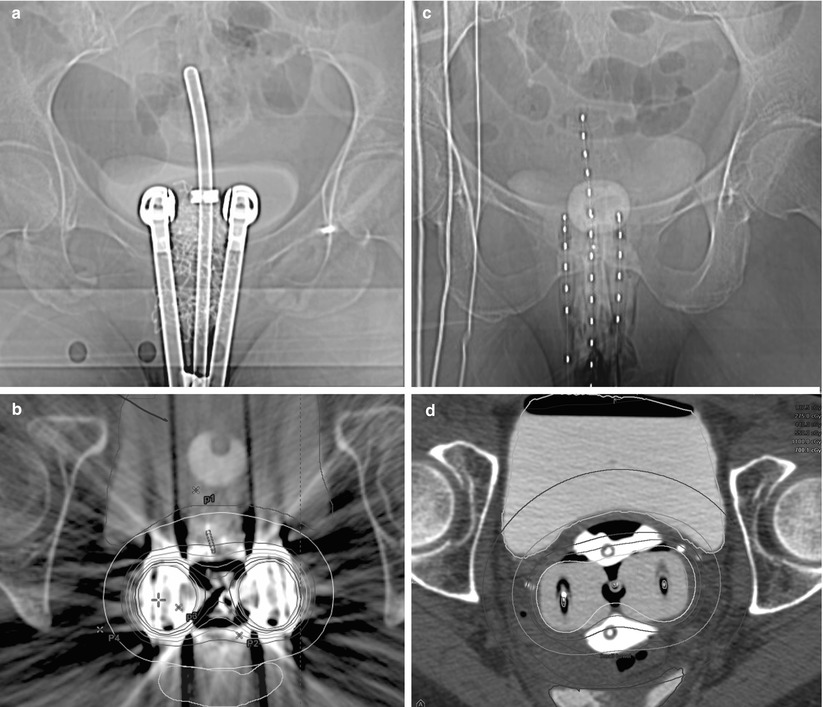
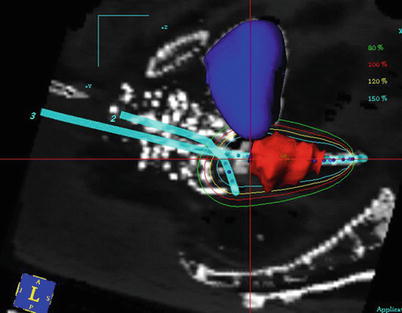

Fig. 26.2
Imaging of intracavitary applicators for cervical brachytherapy. (a) An AP view of a metallic tandem and ovoid applicator with radiation shielding in the ovoids. The tandem is passed through the cervix into the uterus. The ovoids are placed in vaginal fornices. The shielding is intended to attenuate radiation to spare normal structures. (b) An axial view of the same applicator. The metal shielding causes significant artifacts in CT imaging causing difficulties in structure visualization in the vicinity of the ovoids. (c) An AP view of plastic tandem and ovoid applicator. Radio-opaque markers are used to identify the dwell locations within the applicator. (d) An axial view of the same applicator. The lack of metallic shielding allows improved visualization of the tissue surrounding the ovoids

Fig. 26.3
Sagittal plane of a tandem and ovoid implant. The applicator is represented as blue lines, and the dwell locations indicated by blue dots. The target (cervix) has been contoured as red. The bladder has been contoured as blue. Radio-opaque vaginal packing provides additional separation between the applicator and the radiosensitive rectum. The dwell time at each dwell location is varied so the prescription isodose curve (red) encloses the target region to the extent that rectal dose constraints are maintained
The availability of real-time volumetric imaging in the operating room has led to the pursuit of image-guided interstitial brachytherapy. Though widely associated with transrectal ultrasound (TRUS)-guided permanent implants [5], image-guided brachytherapy is being pursued with afterloaded interstitial implants for cervix [8] and prostate cancers [9]. Real-time imaging of the needles with respect to the target region ensures that needle placement will be adequate to achieve the dosimetric goal, and the migration of the radiation treatment planning system to the OR offers the capability of dosimetry-based image-guided brachytherapy.
Sites for Brachytherapy
Breast
Lumpectomy followed by external-beam irradiation of the whole breast is the standard of care for most early-stage breast cancers [10]. Despite excellent outcomes in terms of both local control and cosmesis [11, 12], the typical 6-week duration of the treatment may be problematic to some patients, and a program of accelerated partial breast irradiation (APBI) has been developed. APBI may be delivered using multicatheter implants [13] or by placing an applicator in the lumpectomy cavity [14]. In both instances, imaging is used for placement of the catheters as well as applicator reconstruction and image-based treatment planning. Target definition for treatment differs from most conformal treatment planning because the tumor has been removed with negative margins and the target of the radiation is potential microscopic disease in the surgical cavity. Clips may be placed at the time of lumpectomy to help delineate the region of concern. Inflatable balloon applicators seek to fill the cavity and target the tissue within a specified distance of the balloon surface.
Prostate
The development of prostate-specific antigen (PSA) tests in the 1980s led to the detection of many prostate cancers when they were in the early stage and confined to the gland. These patients are appropriately treated with local therapies including brachytherapy [15]. Brachytherapy treatment outcomes after 5–10 years of follow-up are comparable to those of surgery. Early-stage disease may be treated with permanent implants of low-energy sources such as 125I, 103Pd, and 131Cs under transrectal ultrasound guidance [16–18]. The implant process involves every aspect of image-guided brachytherapy [5]. Implants are planned based on volumetric imaging of the prostate, and the implant is image-guided to match the plan. Post-implant follow-up includes a dosimetric assessment of the implant using CT for image-based brachytherapy evaluation as seen in Fig. 26.4. The approach has been further developed to include MR imaging [19, 20] as well as dosimetry-guided [21] implant procedures including adaptive treatment planning.
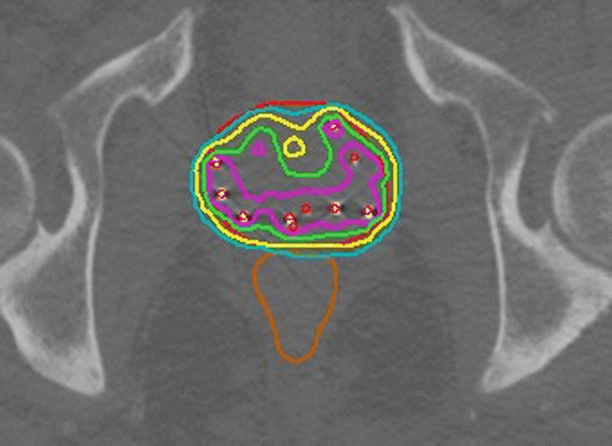

Fig. 26.4
Post-implant analysis of a permanent prostate implant. An axial midgland view of a prostate implant. Permanently implanted sources are readily identified by thresholding and cluster analysis. Red dots indicate source positions as determined by a source-finding algorithm. The source positions are used for dose calculations. The resulting dose distribution is represented by isodose curves. The rapid falloff of dose with the square of the distance from each source implies that any implant will deliver a highly nonhomogeneous dose distribution. An implant may be evaluated by noting the region receiving the prescription dose (green isodose line) or higher. High-dose regions (purple isodose line) occur in the vicinity of implanted sources. While CT easily identifies the sources and bony anatomy, it is relatively difficult to distinguish the soft tissue structures of the pelvis
Gynecologic Brachytherapy
Gynecologic malignancies are well treated with brachytherapy, and local control improves when external-beam radiation is supplemented by brachytherapy [22–24]. The vagina and uterus allow intracavitary placement of applicators and are relatively tolerant of radiation. Implants are temporary using afterloaded sources, and treatments may be delivered by means of low-dose-rate (LDR) sources, delivering dose continuously over the course of days; pulsed-dose-rate (PDR) afterloaders, delivering dose in 10-min intervals hourly; or high-dose-rate (HDR) afterloaders, delivering a few fractions of radiation separated by at least 24 h. Historically these implants have used imaging for evaluation, but there is a recent trend towards image-based conformal plans, and there are recommendations for the use of MR imaging [25, 26]. There are efforts to move beyond image-based planning towards image-guided implants [8].
Imaging Modalities in Brachytherapy
Fluoroscopy
Fluoroscopy is a vital component of many brachytherapy procedures. Planar x-ray images provided a means of verifying placement and/or size of the applicator or provided a general sense of the distribution of brachytherapy needles or sources in the region of the target. While not well suited to distinguishing soft tissues, the difference in attenuation between soft tissue and dense materials makes x-rays well suited to visualizing brachytherapy applicators once placed within the patient. Contrast or radio-opaque material may be used to indicate the location of sensitive structures, such as bladder and rectum in cervix treatments, in the vicinity of the applicators. Geometric distortions make fluoroscopy images inappropriate for dose calculations. Fluoroscopy is useful to guide the placement of brachytherapy catheters and may be used as a supplement to ultrasound imaging in permanent prostate implants to visualize implanted sources within the patient.
Ultrasound
Ultrasound is widely used in brachytherapy procedures for both qualitative and quantitative guidance. Ultrasound offers a non-ionizing imaging modality appropriate for guiding the safe placement of brachytherapy applicators in internal structures. Abdominal transducers are appropriate for the placement of applicators through the cervix, and vascular ultrasound allows appropriate placement of the brachytherapy catheters with respect to vascular lesions.
With some additional equipment, ultrasound can become a volumetric imaging modality. Ultrasound-guided prostate implants use manual or motorized movement of a transrectal ultrasound device to generate an imaging dataset for image-based treatment planning and provide real-time image guidance of needle placement to ensure that the sources are deployed according to the treatment plan [5].
Computed Tomography (CT)
CT imaging is based on the ability of x-rays to distinguish soft tissue from denser materials but is susceptible to imaging artifacts from traditional brachytherapy applicators. CT provides a spatially accurate volumetric dataset appropriate for treatment planning. Manufacturers are now producing brachytherapy applicators that are compatible with CT. Limited soft tissue contrast is a significant problem for image-guided brachytherapy because it is so frequently used in the pelvis. Generally found outside the procedure room, CT provides a means to calculate radiation dose distributions after permanent sources have been placed (image-based evaluation) or plan dose around applicators used for temporary brachytherapy (image-based treatment planning).
Magnetic Resonance Imaging
The soft tissue contrast of MR makes it an extremely appealing imaging modality for brachytherapy. The endometrium, cervix, and prostate are the most common sites for brachytherapy, and in such cases the brachytherapy dose is constrained by neighboring radiosensitive structures that MR can visualize well. MR is subject to geometric distortions, though the relatively small extent of brachytherapy implants may make the distortions acceptable. The GEC-ESTRO recommendations [25] suggest MR for brachytherapy of the cervix. MR imaging for brachytherapy is challenging because metallic or rigid plastic objects are routinely placed in the region of interest, and the optimal pulse sequences differ between visualizing soft tissue and applicators. The use of applicators raises concerns for the use of MR, and care must be taken that all devices are both safe for use in a high magnetic field and compatible with the acquisition of an acceptable MR image. Current trends are for greater use of MR in image-based treatment planning either alone or in conjunction with CT by means of image registration. MR is not generally used for image-guided procedures other than at a few major academic medical centers.
Roles of Imaging in Brachytherapy
Implant Evaluation
Traditionally imaging has been used to produce a model of the geometry of the implanted sources or applicators. The radiation dose delivered by the sources could then be calculated in the coordinate system of the applicator. Radiation dose is reported in terms of an isodose distribution and the dose to reference points representative of anatomic points of interest. Even in ultrasound-guided permanent prostate implants [28], the prototypical image-guided brachytherapy procedure, the implant is evaluated based on post-implant imaging (Figs. 26.1 and 26.4) when there is no ability to affect the implant other than returning to the procedure room to implant additional sources if underdosed regions are detected.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree