Key Points
- •
Secondary hypertrophic osteoarthropathy (HOA) is most often due to malignancies, especially non-small cell lung cancer.
- •
HOA may be due to nonpulmonary conditions.
- •
Bone scan findings of HOA can usually be distinguished from those of metastatic disease.
- •
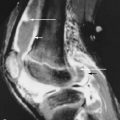
Amyloid arthropathy, which may occur in patients with multiple myeloma, has a distinctive appearance on magnetic resonance imaging.
HYPERTROPHIC OSTEOARTHROPATHY
Definition and Terminology
Hypertrophic osteoarthropathy (HOA) (also called hypertrophic pulmonary osteoarthropathy or Pierre-Marie-Bamberger syndrome ) is a syndrome consisting of clubbing of the fingers and toes; pain and swelling of the distal ends of the limbs; symmetric periosteal reaction, especially of the tubular bones; synovitis; and arthralgia. The disorder has two forms, primary and secondary. The primary form, pachydermoperiostosis or Touraine-Solente-Gole syndrome, is inherited as an autosomal dominant trait with variable expression. The acquired condition is termed secondary hypertrophic osteoarthropathy or pachydermoperiostosis acquisita . The designation “hypertrophic pulmonary osteoarthropathy” has been replaced by hypertrophic osteoarthropathy because nonpulmonary etiologies of the condition have been increasingly recognized.
Etiology
The incidence of HOA is rare in certain countries such as Japan but more common in others. In the United States the incidence has been reported as 0.8%. Up to 90% of cases of HOA are associated with malignancy, usually non-small cell lung cancer. Some of the causes of the secondary form are listed in Box 17-1 .
Pulmonary
Bronchial carcinoma
Secondary lung carcinoma
Mesothelioma
Solitary fibrous tumor of the lung
Pulmonary fibrosis
Empyema
Cystic fibrosis
Chronic infections
Arteriovenous fistulae
Cardiac
Congenital cyanotic heart disease
Infective endocarditis
Mediastinal
Esophageal carcinoma
Thymoma
Achalasia
Liver
Cirrhosis
Liver carcinoma
Biliary atresia
Cholestatic liver disease
Intestinal
Gastrointestinal carcinoma
Inflammatory bowel disease
Chronic infections
Laxative abuse
Polyposis
Whipple disease
Lymphoma
Miscellaneous
Graves’ disease
Thalassemia
Childhood tumors (e.g., nasopharyngeal, lymphoma)
POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, skin changes)
Syphilis
Localized
Aneurysms
Infective arteritis
Vascular prosthesis infection
Patent ductus arteriosus
Hemiplegia
Chronic venous stasis
Takayasu arteritis *
* From Alonso-Bartolome P, Martínez-Taboada VM, Pina T et al: Hypertrophic osteoarthropathy secondary to vascular prosthesis infection: report of 3 cases and review of the literature, Medicine 85:183–191, 2006; Armstrong RD, Crisp AJ, Grahame R et al: Hypertrophic osteoarthropathy and purgative abuse, Br Med J Clin Res Ed 282:1836, 1981; Katsicas M, Ciocca M, Rosanova M et al: Hypertrophic osteoarthropathy in two children with cholestatic hepatic disease, Acta Paediatr 94:1152–1155, 2005; and Kuloğlu Z, Kansu A, Ekici F et al: Hypertrophic osteoarthropathy in a child with biliary atresia, Scand J Gastroenterol 39:698–701, 2004.
HOA is associated with increased blood flow and arteriovenous shunting. Armstrong et al. have summarized the theories explaining the underlying cause(s) of HPOA. A neurogenic cause was first postulated in response to the observation that resolution of HOA could occur with interruption of the vagus nerve even if the tumor remained. The entire spectrum of manifestations of the condition (e.g., facial skin thickening), however, were not adequately explained, and a humoral cause was postulated, such as growth hormone or hepatocyte growth factor. These factors could either be produced by tumor cells and delivered to the periphery, or there could be a failure to remove or deactivate them in the lungs due to arteriovenous shunting. A unifying theory suggests that unfragmented megakaryocytes bypass the pulmonary circulation due to conditions such as cyanotic heart disease and stimulate the endothelium to produce platelet-derived growth factors (PDGF) and vascular endothelial growth factor (VEGF). These lead to angiogenesis, endothelial hyperplasia, clubbing, and HOA. It is postulated that if the cause of HOA is carcinoma-derived growth factor, tumor resection will lead to improvement, whereas if shunting is prominent (fewer cases), tumor resection will not ameliorate the symptoms.
The cause of HPOA in chronic cholestatic liver disease is uncertain, but resolution can occur with improvement in liver disease or after liver transplantation.
Clinical Features
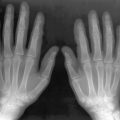
Clubbing of the fingers and toes is the most frequent manifestation of HOA. Periosteal reaction of the long bones is common. Skin thickening may develop that is so prominent that ridges and furrows resembling the gyri of the brain are seen on the scalp and forehead. These features may be confused with acromegaly. Synovitis with noninflammatory fluid is a recognized manifestation, but inflammatory arthritis has also been documented. Armstrong et al. reported two patients with inflammatory synovitis who presented with several months of swelling of the wrists and ankles, elbow and shoulder, and clubbing of the fingers and toes. Both patients were found to have periosteal reaction, elevated sedimentation rates, and lung tumors (small cell in one and non-small cell in the other).
Clubbing, symmetric periosteal reaction, and arthritis are typical manifestations of HOA.
An interesting presentation is seen in patients with infected vascular grafts where the findings of HOA are localized to the areas distal to the vascular prosthesis.
Imaging Findings
The clinical manifestations should lead to imaging evaluation. An underlying pulmonary lesion can be detected using standard radiography and computed tomography (CT). Care must be taken because pulmonary infections and benign lesions and malignancies may result in HOA and even in uptake on fluorodeoxyglucose positron emission tomography (FDG-PET) scanning. Therefore tissue diagnosis is necessary.
Radiographs
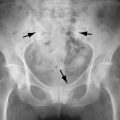
The disorder is characterized on radiographs by the presence of periostitis that usually involves the diaphyses of long tubular bones ( Figures 17-1 and 17-2 ). Although periosteal reaction can be seen on radiographs, these are less sensitive than bone scan or magnetic resonance imaging (MRI) for early diagnosis. Periosteal reaction is seen first along the proximal and distal shafts and metaphyses of long tubular bones as a single layer. As the condition progresses, the periosteal reaction becomes more marked, extends to the epiphysis, and becomes laminated or multilayered. Eventually it can involve all of the tubular bones, causing increased cortical thickness and an irregular surface. The interosseous membranes can ossify.








The periosteal reaction becomes thicker and more extensive with longer disease duration. Comparison by Pineda et al. of patients with cyanotic heart disease to those with lung cancer showed that those with congenital cyanotic heart disease (CCHD) or primary HOA demonstrated periosteal new bone formation along the diaphyses, metaphyses, and epiphyses whereas epiphyseal involvement was not seen in patients with HOA secondary to lung carcinoma. Also, the configuration of the periosteal reaction seems to be related to disease duration. Thus, in the same study, periosteal reaction in primary HOA and in CCHD was usually multilayered and an irregular pattern was seen in one third of those patients ( Figure 17-3 ). In the patients with lung cancer–related HOA, the most frequent appearance was a single layer of periosteal new bone and no irregular periostitis was seen.